Background: Hyperbaric oxygen therapy (HBOT) appears to be of benefit as an adjuvant treatment for patients post cerebrovascular accident (CVA).
Aim of the work: We conducted a study to determine whether Hyperbaric Oxygen Therapy (HBOT) is of benefit, in adults post Cerebrovascular Accident (CVA) and whether there are important differences in outcome associated with this method of treatment regarding early outcome after completion of treatment and later outcome 2 months after start of treatment.
Methods: In a randomized controlled trial, thirty patients post Cerebrovascular Accident (CVA) were enrolled in the study and divided into two groups. Group I (HBOT group): Included 15 patients who were assigned to HBOT through oronasal mask in multi-place hyperbaric chamber (ETC Bara-Med, 1.5 ATA, 60 minutes per session, for 20 sessions) plus conventional therapy. Group II (Control group): included 15 patients who were assigned to standard conventional therapy alone. Both groups were compared regarding following parameter: NIHS score, MOCA score & SIS score at primary evaluation of both groups, after completion of 20 sessions in HBOT group and after 1 month in control group and after 2 months in both groups. Both groups are compared regarding significant difference in Physical function, mental function and effect on quality of life.
Results: Compared with standard conventional therapy, hyperbaric oxygen therapy (HBOT) was associated with early greater mean improvement regarding to NIHS score (2.7 vs. 7.0, P-value 0.03), MOCA score (23.7 ± 7.8 vs. 17.4 ± 8.3, P-value 0.04) and SIS score (67.4 ± 10.4 vs. 53.9 ± 17.7, P-value 0.03). These improvement continue after discontinuation of treatment with hyperbaric oxygen therapy with late mean scores in NIHS (1.8 vs. 5.9, P-value 0.04), MOCA (24.6 ± 8 vs.18.4 ± 8, P-value 0.05) and SIS scores (69.6 ± 8.3 vs. 56.4 ± 17.6, P-value 0.04) with significant difference between patient receiving adjuvant hyperbaric oxygen therapy (HBOT) with conventional therapy and patient receiving conventional therapy alone.
Conclusions: For patients post Cerebrovascular Accident (CVA), Hyperbaric Oxygen Therapy (HBOT) as an adjuvant treatment to conventional treatment, safely provides improvement of physical functions, cognitive functions, and quality of life. We recommend that hyperbaric oxygen therapy could be used in patient with (CVA), as an adjuvant treatment to other modalities of treatment, in post stroke stage with residual physical, cognitive function deficits and impairment of normal quality of life.
Hyperbaric Oxygen Therapy (HBOT) defined by Undersea and Hyperbaric Medical Society (UHMS), as an intervention in which an individual breathes near 100% oxygen intermittently while inside a hyperbaric chamber that is pressurized to greater than sea level pressure or (1 atmosphere absolute [ATA] or 101.3 kilopascals [KPa]) and for clinical purposes, the pressure must equal or exceed 1.4 ATA (141.8 kPa) while breathing near 100% oxygen. [1]
The current World Health Organization (WHO) definition of stroke introduced in 1970 and still used is; “Rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin.” [2]
Cerebrovascular Accident (CVA) especially acute ischemic stroke is a leading cause of mortality, long-term disability worldwide and always ranked at the top causes of death and most of hospitalized acute CVA patients have ischemic stroke. [3] Although the mortality rate of ischemic stroke is less than that of hemorrhagic stroke, [4] it still results in patient disabilities and complications that often lead to significant costs to individuals, families, and society. Thus, searching for an effective supplemental treatment for CVA and residual complication is imperative. [5]
Hyperbaric Oxygen Therapy (HBOT) is approved by American Food and Drug Administration (FDA) and recommended by Undersea And Hyperbaric Medical Society (UHMS) for use in treatment of many neurological disorders [1] as; Cerebral and Spinal Decompression Sickness (DCS), [6] cerebral air or gas embolism (CAGE), [7] Carbon Monoxide Poisoning (COP), [8] sudden loss of vision with Central Retinal Artery Occlusion (CRAO), [9] sudden deafness with Idiopathic Sudden Sensorineural Hearing Loss (ISNHL), [10] Non-Surgical Intracranial Abscess (ICA), [11] Delayed Radiation Injury (DRI) as radiation myelopathy and encephalopathy, [12] European Committee of Hyperbaric Medicine (ECHM) recommended also approved neurological disorders as stage IV neuroblastoma and brain injury in highly selected patients as acute and chronic traumatic brain injury, post anoxic encephalopathy and chronic stroke. [13]
After acute cerebral ischemia, a complicated cascade of biochemical events occurs including inflammation, an [20, 21] increased production of free radicals and Reactive Oxygen Species (ROS) in the tissue and plasma, increased platelet activation and platelet-leukocyte interactions, inflammatory cell adhesion molecule production, firm adhesion, and transmigration of leukocytes along the vessel wall. These events finally contribute to endothelial and neurological dysfunction. [14]
Years of clinical experience revealed that the dramatic spontaneous recovery from stroke occurs mainly within the first 30 days, though moderate and severe stroke survivors continue to improve for at least 90 days. [15] Most of the recovery involves brain regions rendered dysfunctional, but not dead. [16] Accumulated data from visualizations of these nonactive (stunned) regions indicates that they may persist alive but dysfunctional for months, even years, after the acute injury. [17,18]
It was proposed that the oxygen supply to these under-active neurons was low due to stroke damage to blood vessels in these regions, leading to oxygen deficiency, anaerobic metabolism and ATP depletion. Decreased oxygen level not only causes reduction in the neuronal activity but also prevents angiogenesis to replace the stroke-damaged blood vessels and the generation of new synaptic connections and high oxygen supply is essential for repair of the penumbra or stunned regions. [19]
Intensive functional therapy and rehabilitation for post stroke patients are considered essential for maximizing the patients’ quality of life but unfortunately, these programs are often just partially successful, and additional therapeutic approaches towards metabolic recovery of affected cerebral tissues are needed.
Hyperbaric Oxygen Therapy (HBOT), which combines the action of hyperoxia and hyperbaric pressure, leads to significantly improved tissue oxygenation and could restore neuronal activity in metabolically dysfunctional areas as an adjunctive therapy for the treatment of patients with ischemic stroke, [22] and potential mechanisms of HBOT include improving cerebral blood flow, initiating vascular repair, restoring the functional blood brain barrier, reducing inflammatory reactions and brain edema and these events finally can activate neuroplasticity, effectively evoking neuronal repair and revitalize chronically impaired brain functions, in the chronically metabolic dysfunctional stunned areas during poststroke phase. [23]
Aim of the workWe conducted a study to determine whether Hyperbaric Oxygen Therapy (HBOT) as an adjuvant therapy to standard conventional treatment, is of immediate benefit (early outcome) in adult patients with Cerebrovascular Accident (CVA), compared to conventional therapy alone, and whether there are important differences in late outcome associated with this method of treatment, through investigation of the effects of HBOT on physical functions, cognitive functions and quality of life, in adult patients immediately post (CVA) cerebrovascular accident
The study was conducted in the Naval Hyperbaric Medical Institute (NHMI) of the military medical academy of Egypt, during two years of study period (2018- 2019), thirty adult patients with a diagnosis of cerebrovascular stroke presented to Naval Hyperbaric Medical Institute (NHMI), were included in our study and all patients informed about the study method in details and a written high risk informed consent for treatment obtained from all patients and or families.
Inclusion criteriaAdult patients of any gender with age more than 18 years and less than 80 year with clinical diagnosis of cerebrovascular accident (ischemic or hemorrhagic stroke) within 6 months before primary evaluation in naval hyperbaric medical institute were included in the study. All patients were fulfilling the following inclusion criteria; Clinical picture of cerebrovascular accident, radiological findings of cerebrovascular accident by brain computed tomography (CT), must be able to equalize ears and willing to complete outcome measures.
Exclusion criteriaThe following patients are excluded from the study; Patients Glasgow coma scores (GCS) less than 13, Patients with traumatic brain injury (TBI), Claustrophobia (patient who is unable to be enclosed inside the chamber), Inability to equalize ears, Type C Tympanogram patients, Inability to protect airway, or requiring frequent suctioning, Pregnant (beta HCG will be assessed in women in childbearing period), Severe psychiatric disorders such as schizophrenia or bi-polar disorders, Degenerative mental diseases as Alzheimer's disease or senile dementia, Heart failure patients with ejection fractions less than 50%, Patients with active malignancy, Patients with emphysema with CO2 retention, Pneumothorax, Seizure disorder and patients with uncontrolled high fever.
Study design and techniquesThe study was prospective randomized controlled trial with two treatment groups (Two arms), Hyperbaric Oxygen Therapy Group (HBOT) and Control group (Control).
Group one, HBOT group (n=15): fifteen adult patients with Cerebrovascular Accident (CVA) will receive conventional medical treatment according to American Stroke Association guidelines ASA (as thrombolytic therapy, antiplatelets, physiotherapy or surgical intervention) with adjunctive HBOT).
HBOT group will receive 20 sessions of HBOT at 1.5 ATA for 60 minutes in a multi-place hyperbaric chamber (American ETC Biomedical systems BARA-MED Model 6/2/6) pressured with compressed air, whereby patients will breath 100% oxygen through face mask, 5 days per week for four weeks Compared with.
Group two, control group (n=15): fifteen adult patients with Cerebrovascular Accident (CVA) will receive conventional treatment alone according to American Stroke Association guidelines ASA.
The baseline clinical characteristics were similar in both groups.
All included patients were subjected to the following: Detailed medical history; age, gender, smoking, diabetes mellitus, hypertension, cardiovascular disease, COPD and history of fits, Clinical examination; Neurological assessment use NIHSS score, SIS score, MOCA score and Chest, cardiology and ENT examination, CT brain, Random blood sugar, Electrocardiogram(ECG), Echocardiography and Tympanogram.
Evaluations measuresPretreatment evaluation, all patients had been evaluated by National Institute of Health Stroke Scale (NIHSS), Montreal Cognitive Assessment score (MOCA) and Stroke Impact Scale (SIS) when patient primary evaluated in Naval Hyperbaric Medical Institute (NHMI),
Secondary evaluation, patients in the HBOT group had been evaluated by NIHSS, MOCA and SIS after 20 sessions of HBOT. The control group will be evaluated by NIHSS, MOCA and SIS 30 days after primary evaluation,
Third evaluation, one month after secondary evaluation, all patients had been evaluated third evaluation again using NIHSS, MOCA and SIS.
The clinical response is evaluated by comparing the patient’s pre-treatment NIHSS, MOCA and SIS scores with those evaluated after 20 sessions of HBOT in the HBOT group. Similarly, NIHSS, MOCA and SIS scores of patients not receive HBOT will be compared to those 30 days after primary assessment in control group.
To determine the HBOT efficacy, changes of NIHSS, MOCA and SIS scores between the HBOT and control groups were demonstrated by;
Early efficacy, where changes in NIHSS, MOCA and SIS scores at the time before HBOT and after 20 sessions of HBOT in HBOT group will be compared to changes of scores between primary assessment and 30 days after primary assessment in the control group, and
Late efficacy, where changes of NIHSS, MOCA and SIS scores between pretreatment and 2 months post treatment in the HBOT group will be compared with changes of scores between the primary assessment and 2 months after primary assessment in the control group.
Analysis methodThe sample was selected by simple random sample and all members of population have an equal chance of being selected as part of the sample.
Every patient with Cerebrovascular Accident (CVA) presented to the Naval hyperbaric medical institute and matching the inclusion and exclusion criteria is included in our study, data collection using textual, tabular and graphical method. Variables in both groups are identical with the single exception of one variable (HBOT) in hyperbaric oxygen therapy treated group. Data analysis on presentation, 30 days and 60 days later P-value (proportional value) determine the level of significance with type I error, P-value of equal or less than 0.05 was considered to indicate statistical significance and this significance become more and more with less value of p value.
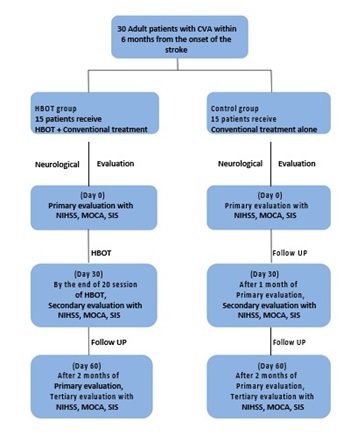
Figure 1: Scheme of present study (Shehata I, Hamdy M, Gamal Aldin A, Abd Elradi E, et al. (2019) Hyperbaric Oxygen Therapy Versus Conventional Therapy as an Adjunctive Treatment for patient Post Cerebrovascular Accident, Naval hyperbaric medical institute (NHMI), Military medical academy (MMA), Egypt.
In a randomized controlled trial, 30 patients, 7 (23.3%) females and 23 (76.7%) males with cerebrovascular accident (CVA) within 6 month of stroke onset (mean duration from stroke onset 2.6 months), were enrolled in the study with mean age is 50.7 ± 12 years. Patient was divided randomly into two groups; Hyperbaric Oxygen Therapy Group (HBOT) and Control group:
Group two (Control group): 15 adult patients with cerebrovascular accident (CVA) received only conventional treatment
Comparison of baseline characteristics of HBOT group and Control group, regarding age and gender:There were no statistically significant differences in baseline characteristics among the two groups regarding age and gender, table (1) and figure (2).
Table 1: Demographic data of the patients at primary evaluation.
|
Characteristic |
HBOT (N = 15) |
Control (N = 15) |
P- value |
|
Age (yrs) |
48.2 ± 14.3 |
53.0 ± 9.09 |
0.26 |
|
Male gender % |
73% (11) |
80% (12) |
0.33 |
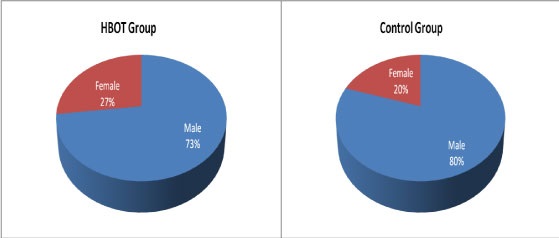
Figure 2: Demographic data of the patients
Group one (HBOT group): 15 adult patients with cerebrovascular accident (CVA) received hyperbaric oxygen therapy (HBOT) with conventional treatment, and
Group one: 15 adult patients (11 male and 4 females with mean age 48 years).
Group two: 15 adult patients (12 male and 3 females with mean age 53 years).
Comparison of baseline characteristics of HBOT group and Control group, regarding duration since onset and type of cerebrovascular accident (CVA):There were no statistically significant differences in baseline characteristics among the two groups regarding duration from onset of stroke and major type of CVA, with mean duration from stroke till primary evaluation is 2.9 months in HBOT group one and 2.3 months in Control group. 73% had ischemic stroke in group one and 80% in group two, table (2) and figure (3 and 4).
|
Characteristic |
HBOT (N = 15) |
Control (N = 15) |
P- value |
|
Duration from onset of CVA |
Mean 2.9 m |
Mean 2.3 m |
0.28 |
|
Type of CVA |
|
|
|
|
a. Ischaemic |
73% (11) |
80% (12) |
0.33 |
|
b. Haemorrhagic |
27% (4) |
20% (3) |
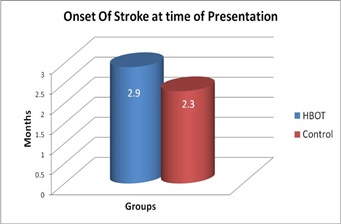
Figure 3: Duration from Onset of CVA among the patients.
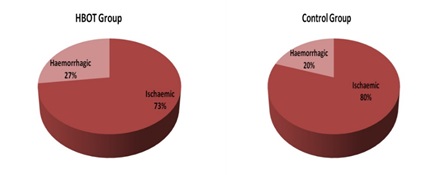
Figure 4: Type of CVA among the patients.
There were no statistically significant differences in baseline characteristics among the two groups regarding history of medical illness, table (3) and figure (5).
|
Characteristic |
HBOT (N = 15) |
Control (N = 15) |
P- value |
|
Hypertension % |
60% (9) |
53% (8) |
0.33 |
|
Diabetes mellitus % |
33% (5) |
26% (4) |
0.33 |
|
Ischemic heart disease % |
6% (1) |
13% (2) |
0.33 |
|
Valvular heart disease % |
20% (3) |
13% (2) |
0.33 |
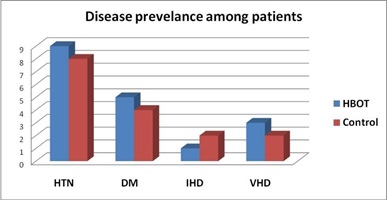
Figure 5: Diseases prevalence among the patients.
Comparison between HBOT Control group, regarding primary evaluation: Group and NIHSS at regarding NIHSS at primary evaluation, table (4) and figure (6). There were No statistically significant differences between the two groups.
Comparison between HBOT group and Control group, regarding MOCA score at primary evaluation:|
Category |
HBOT (N = 15) |
Control (N = 15) |
P- value |
|
1. Visuospatial & Executive |
2.6 |
2.26 |
0.61 |
|
2. Naming |
1.73 |
2 |
0.53 |
|
3. Recall |
1 |
2.13 |
0.09 |
|
4. Attention |
3.46 |
3.4 |
0.93 |
|
5. Language |
1.73 |
1.8 |
0.88 |
|
6. Abstraction |
1 |
1.13 |
0.76 |
|
7. Orientation |
3.73 |
4.06 |
0.7 |
|
Total Score |
15.2 ± 9.4 |
16.5 ± 8.3 |
0.73 |
There were no statistically significant differences between the two groups regarding SIS score at primary evaluation, table (6) and figure (6).
|
Category |
HBOT (N = 15) |
Control (N = 15) |
P- value |
|
1. Level of consciousness (LOC) |
0.73 |
0.73 |
1 |
|
2. Best Gaze |
0.06 |
0.13 |
0.33 |
|
3. Visual Field |
0.06 |
0.4 |
0.09 |
|
4. Facial Palsy |
0.8 |
1 |
0.38 |
|
5. Motor Affection |
4.8 |
3 |
0.15 |
|
6. Sensory Affection |
0.33 |
0.33 |
1 |
|
7. Coordination |
0.06 |
0.2 |
0.16 |
|
8. Aphasia |
0.73 |
0.6 |
0.63 |
|
9. Dysarthria |
0.8 |
0.86 |
0.33 |
|
10. Extinction and Inattention |
0.26 |
0 |
0.1 |
|
Total Score |
8.7 ± 6.5 |
7.1 ± 5.4 |
0.48 |
|
Category |
HBOT (N = 15) |
Control (N = 15) |
P- value |
|
1. Dressing |
2.66 |
3.13 |
0.32 |
|
2. Bathing |
2.8 |
3 |
0.68 |
|
3. Toileting |
3.26 |
3.13 |
0.82 |
|
4. Bladder control |
4.46 |
4.26 |
0.33 |
|
5. Bowel control |
4.4 |
4.46 |
0.33 |
|
6. Standing |
3.26 |
3.33 |
0.71 |
|
7. Shopping |
2.86 |
2.93 |
0.81 |
|
8. Household chores |
2.86 |
3.06 |
0.67 |
|
9. Sitting |
3.33 |
3.53 |
0.76 |
|
10. Walking |
2.93 |
3.33 |
0.55 |
|
11. Move from bed to chair |
3.26 |
3.13 |
0.85 |
|
12. Walk Fast |
2.86 |
2.53 |
0.06 |
|
13. Climb stairs |
2.8 |
2.73 |
0.58 |
|
14. Walk one block |
2.86 |
3.1 |
0.06 |
|
15. Get in & out of car |
3 |
3.2 |
0.78 |
|
16. Carry heavy objects |
2.86 |
2.93 |
0.58 |
|
Total Score |
49.6 ± 20.6 |
51.8 ± 18.9 |
0.79 |
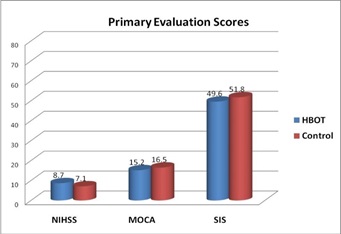
Figure 6 NIHSS, MOCA and SIS Scores at primary evaluation.
There were no statistically significant differences in management between the two groups (only one variable is HBOT 1.5 ATA, 5 days per week for 20 sessions in (Multi-place BaraMed ETC chamber Model 6/2/6) that patient receive in group one. Both groups receive similar treatment according to stroke management guidelines of ASA and all patients were treated with physiotherapy.
Early outcome measuresHyperbaric oxygen therapy (HBOT) was associated with greater improvement in NIHS score, MOCA score and SIS score, than conventional treatment alone in treatment of cerebrovascular accident (CVA).
Mean changes 30 days after treatment: Comparison between HBOT group and Control group, regarding mean changes in NIHSS after 30 days: Comparison of NIHSS in group I that was done 30 days after initiation of HBOT (after 20 session of HBOT) with group II that was done 30 days after primary evaluation, that use conventional treatment alone, showed significant reduction in NIHSS in HBOT group than control group, table (8) and figures (7,10).
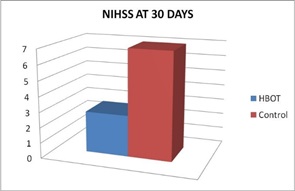
Figure 7: NIHSS Scores of two groups at 30 Days
Comparison between HBOT group and Control group, regarding mean changes in MOCA score after 30 days: Comparison of MOCA score in group I that was done 30 days after initiation of HBOT (after 20 session of HBOT) with group II that was done 30 days after primary evaluation, that use conventional treatment alone, showed significant increase in MOCA score in HBOT group than control group, table (9) and figures (8,10).
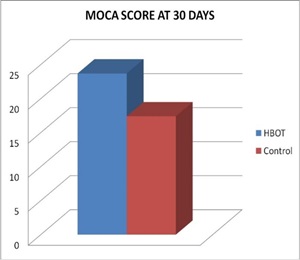
Figure 8: MOCA Score of two groups at 30 Days.
Comparison between HBOT group and Control group, regarding mean changes in SIS score after 30 days: Comparison of SIS score in group I that was done 30 days after initiation of HBOT (after 20 session of HBOT) with group II that was done 30 days after primary evaluation, that use conventional treatment alone, showed significant increase in SIS score in HBOT group than control group, table (10) and figures (9,10).
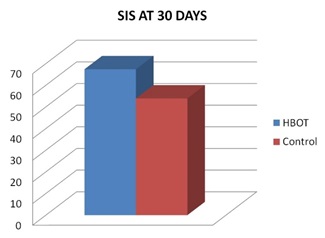
Figure 9
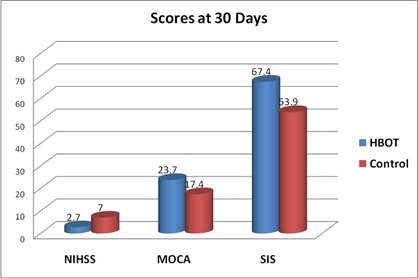
Figure 10
Comparison between HBOT group and Control group, regarding mean changes in NIHSS score after 60 days: Comparison of NIHSS score in group I that was done 60 days after initiation of HBOT with group II that was done 60 days after primary evaluation, that use conventional treatment alone, showed significant reduction in NIHSS score in HBOT group than control group, table (11) and figure (11).
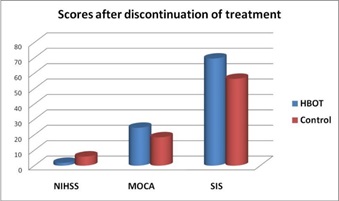
Figure 11: NIHSS, MOCA and SIS Scores of two groups at 60 Days.
Comparison between HBOT group and Control group, regarding mean changes in MOCA score after 60 days: Comparison of MOCA score in group I that was done 60 days after initiation of HBOT with group II that was done 60 days after primary evaluation, that use conventional treatment alone, showed no significant changes in MOCA scores between HBOT group and control group, table (12) and figure (11).
Comparison between two groups regarding mean changes in SIS score after 60 days and discontinuation of treatment: Comparison of SIS score in group I that was done 60 days after initiation of HBOT with group II that was done 60 days after primary evaluation, that use conventional treatment alone, showed significant increase in SIS score in HBOT group than control group, table (13) and figure (11).
Evaluation of clinical response in each group: NIHSS changes in HBOT group and NIHSS changes in control group: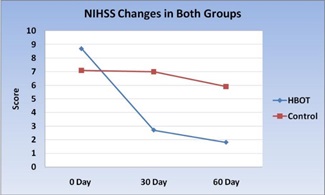
Figure 12: NIHS score changes in HBOT group and Control group
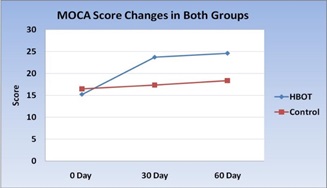
Figure 13: MOCA score changes in HBOT group and Control group.
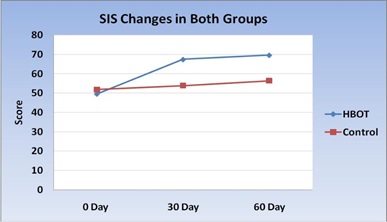
Figure 14: SIS score changes in HBOT group and Control group
The purpose of the present study was to investigate the role of Hyperbaric Oxygen Therapy (HBOT) as an adjunctive therapeutic intervention for stroke patients.
Cerebrovascular Accident (CVA) or stroke is the second most frequent cause of death after coronary artery disease, accounting for 6.3 million deaths worldwide (11% of the total). About 3.0 million deaths resulted from ischemic stroke while 3.3 million deaths resulted from hemorrhagic stroke. Overall, two thirds of strokes occurred in those over 65 years old. [24] In the past 40 years, the stroke morbidity fell by 42% in developed countries, while rose in developing nations. Ischemic stroke occupies nearly 80% of stroke, which is a heavy burden of the patient’s family and the society. A stroke may result in a variety of functional deficits including physical, cognitive, and quality of life impairments. However, the treatments for stroke are still limited. [25] Accumulated evidences demonstrate that, stability of oxygen saturation is critical for neuronal injury after stroke. Local hypoxia and anoxia result in cellular damage. [26] HBO has been shown to facilitate oxygen delivery, increase oxygen supply, ameliorate cerebral circulation, decrease cerebral edema, reduce brain infarction and utilized to treat cerebral ischemia. [27] Over the past decades, there has been a steady stream of published clinical trials and reviews investigating the effectiveness of Hyper Baric Oxygen Therapy (HBOT) on Cerebrovascular Accident (CVA).
Despite decades of interest in HBOT, previous studies investigating the effects of HBOT following a stroke have produced mixed results and recent studies as; Emily R. Rosario et al., 2018, studied the effect of HBOT on functional impairment caused by ischemic stroke,[28] Chen Yu Chen, et al., 2018, studied increased circulating endothelia progenitor cells and improved short term outcomes in acute non cardio embolic stroke after hyperbaric oxygen therapy, [29] Bennett MH et al., Cochrane Database of Systematic Reviews, 2014, studied hyperbaric oxygen therapy for acute ischemic stroke, [30] Efrati S et al., 2013, founded that hyperbaric oxygen can induce late neuroplasticity in post stroke patients, [31] Cheng-Hsin Chen et al., 2012, studied effects of repetitive hyperbaric oxygen treatment in patients with acute cerebral infarction, the results of these studies will be discussed later. [32] While research suggests a benefit of HBOT for neurologic injury, controlled clinical trials have not been able to clearly define the benefits[28].
The present study was conducted study to determine whether HBOT as an adjuvant treatment, is of benefit in adults with cerebrovascular accident and whether there are important differences in outcome associated with this method of treatment regarding physical function, cognitive function and quality of life.
Previous studies of the last 10 years were reviewed, that included patients with cerebrovascular accident and received HBOT with standard treatment, and compared their result with the results of the present study. The main outcome measures are HBOT effect on physical function, cognitive function and quality of life in patient with cerebrovascular stroke [28-32].
In the present study, 30 patients with cerebrovascular and within 6-month duration post CVA had been enrolled in the study (mean duration is 2.6 months).
In the present study, the sample size is larger than sample size of Emily R. Rosario et al., 2018, study (n = 30 vs 7), and near similar to the sample size of HBOT group in Cheng-Hsin Chen et al., 2012, study (n = vs 16). [28, 32]
In the present study, the duration from the onset of the stroke till primary evaluation of the patient to be enrolled in the study was 6 month in chronic post stroke stage, with (mean time from onset of stroke is 2.6 months), Emily R. Rosario et al., 2018, select the patient within 12 month of ischemic stroke, [28] S. Efrati et al., 2013, select patient in chronic stage from 6-36 months since stroke onset[132] while Cheng-Hsin Chen et al., 2012, select the patient 3-5 days from the onset of stroke that affect the results. [32]
In the present study, the patients with the following criteria are excluded from the study; patients Glasgow Coma Scores (GCS) less than 13 at the time of primary evaluation for HBOT, patients with Traumatic Brain Injury (TBI), claustrophobia (patient unable to be enclosed with the chamber), inability to equalize ears, type C tympanogram patients, inability to protect airway, and or requiring frequent suctioning, pregnant , severe psychiatric disorders, degenerative brain diseases as Alzheimer disease, heart failure patients with ejection fractions less than 50%, patients with active malignancy, emphysema with CO2 retention, pneumothorax, seizure disorder or uncontrolled high fever.
In the present study, the patients were divided into two groups; Group one, HBOT group (n = 15): fifteen adult patients with cerebrovascular accident CVA who received conventional medical treatment according to American Stroke Association Guidelines ASA ( as thrombolytic therapy, anti-platelets, physiotherapy or surgical intervention ) with adjunctive Hyperbaric Oxygen Therapy (HBOT) in the form of 20 sessions of HBOT at 1.5 ATA and for one hour in a hyperbaric chamber pressured with compressed air, whereby patients would breath 100% oxygen, 5 days per week. Compared with group two, control group (n = 15): fifteen adult patients with cerebrovascular accident CVA would receive conventional treatment alone according to American stroke association guidelines ASA.
This protocol of treatment is similar to S Efrati et al., 2013, who used HBOT at 2 ATA for 40 sessions and indicated that HBOT above 2 ATA may have undesirable neurofunctional inhibition and even focal toxicity, [31] and in Emily R. Rosario et al., 2018, each candidate received HBOT for a total of 40 session 90-minute treatments over a 12-week period. [28]
The differences in protocols of treatment are related to the local protocols used by different chambers in different centers.
Early efficacy (outcome), where changes in scores at the time before HBOT and after 20 sessions of HBOT in HBOT group compared to changes of scores between primary assessment and 30 days after primary assessment in the control group, and late efficacy (outcome), where changes of scores between pretreatment and 2 months post treatment in the HBOT group compared with changes of scores between the primary assessment and 2 months after primary assessment in the control group.
In the present study, the baseline characteristics of the patients on primary evaluation, are similar in both treatment groups (cerebrovascular stroke received Hyperbaric Oxygen Therapy (HBOT) with conventional treatment, and cerebrovascular stroke received only conventional treatment alone) regarding age (48.2 ± 14.3, versus 53.0 ± 9.09 with P value = 0.26) , gender (male gender 73% versus 80%), vascular risk factors on presentation (hypertension, diabetes mellitus, ischemic heart disease, and valvular heart disease) and type of stroke ( ischemic stroke 11 versus 12), and there is no statistically significant difference between both groups.
In the present study, the intervention was correctly delivered using multi-place hyperbaric chamber (American ETC Bara-Med Model 6/2/6) with 1.5 ATA for 60 minutes, 5 days per week, for 20 sessions that similar to those in previous studies. HBOT treatment group was associated with discomfort with some patients (26%) due to tight oronasal mask, pressure over ears or claustrophobia and this was avoided by training for equalization of pressure around the ears and good communication and this did not prevent the patient from completion of treatment. In the present study regarding primary evaluation, NIHSS, MOCA and SIS scores show no significant difference in the degree of disabilities between HBOT group and control group, regarding physical, cognitive functions and quality of life.
In the present study regarding physical parameters scores (NIHSS), HBOT plus conventional treatment, compared with conventional treatment alone, was associated with early greater improvements in mean NIHSS score (2.7 vs. 7.0, P-value 0.03) and was recorded after completion of 20 sessions of HBOT.
Cheng-Hsin Chen et al., 2012, studied effects of repetitive hyperbaric oxygen treatment in patients with cerebral infarction. This prospective study assessed the efficacy and safety of HBOT as adjuvant treatment for ischemic stroke in patients who did not receive thrombolytic therapy. The HBOT group (n=16) received conventional medical treatment with 10 sessions of adjunctive HBOT within 3-5 days after stroke onset, while the control group (n=30) received the same treatment but without HBOT. Early (two weeks after onset) and late (one month after onset) outcomes (NIHSS scores) and efficacy (changes of NIHSS scores) of HBOT were evaluated. Both early and late outcomes of the HBOT group showed significant difference. In the control group, there was only significant difference in early outcome. For early efficacy, there was no difference when comparing changes of NIHSS scores between the two groups but there was statistically significant difference when comparing changes of NIHSS scores at one month and the study concluded that HBOT may be effective for patients with acute ischemic stroke and is a safe and harmless adjunctive treatment. [32]
In the present study, the findings were in line with the literature as we did observe significant effects on some domains after one month of starting treatment with HBOT, as improvement of the degree of facial weakness but there were also a number of areas where no significant change were observed. Overall physical function continues to improve (1.8 vs. 5.9, P-value 0.04) and HBOT effect starts to appear in other domains as degree of weakness of upper and lower extremities, after one month of completion of treatment with HBOT.
Bennett MH et al., 2014, Cochrane systematic reviews, an update of Cochrane review 2005, assessed the effectiveness and safety of adjunctive HBOT in the treatment of people with acute ischemic stroke in randomized controlled trials that compared the effects of adjunctive HBOT versus those of no HBOT (no treatment or sham). A systematic review of 7 of the 11 randomized trials showed no significant difference was observed in the mortality at 6 months in the HBO treated group compared with the control group. However, neurological function scale scores were improved with HBO therapy. For example, the mean Orgogozo Scale score was higher and the mean Trouillas Disability Scale score lower following HBO treatment than that of the control group. They concluded that, although current evidences are not enough to offer explicit guideline for clinical medicine, the possible value of HBO in clinical practice are not excluded. [30]
In the present study, early significant improvements in cognitive function were observed following HBOT with MOCA scores (23.7 ± 7.8 vs. 17.4 ± 8.3, P-value 0.04) including visuospatial and executive functions, naming, recall, attention, language, abstraction and orientation. Although some late clinical response occurs in control group, there was significant improvements one month after discontinuation of treatment between HBOT group and control group regarding MOCA score (24.6 ± 8 vs.18.4 ± 8, P-value 0.05).
Emily R. Rosario et al., 2018, Each candidate (n=7) received HBOT for a total of 40 session 90-minute treatments over a 12-week period, they found improvements in cognition and executive function as well as physical abilities, specifically, improved gait. Participants reported improved sleep and quality of life following HBOT treatment. They also saw changes in serum levels of biomarkers for inflammation and neural recovery (TNF- α and NSE). The functional domains where improvements were observed following HBOT treatment, the improvements were maintained up to 3 months following the last treatment. However, the physiological biomarkers showed a pattern of more transient changes following HBOT treatment. Findings from this study support the idea of HBOT as a potential intervention following stroke. [28]
Social isolation is very common with a long-term disability along with significant loss of self-worth, income, independence, and many other domains, thus a sustained increase in global improvement, participation, and emotional wellbeing are of particular importance. Recovery after stroke correlates with penumbra area (non -active or stunned brain regions), which may persist for years. [31]
In the present study, early improvement of quality of life and overall global recovery, were observed following HBOT as measured with the SIS score (67.4 ± 10.4 vs. 53.9 ± 17.7, P-value 0.03). These treatment effects were maintained when examined every month following treatment (69.6 ± 8.3 vs. 56.4 ± 17.6, P-value 0.04).
Efrati S et al., 2013, evaluated whether increasing dissolved oxygen by HBOT could activate neuroplasticity in patients with chronic neurologic deficiencies due to stroke. A prospective, randomized, controlled trial including 74 patients and all participants suffered a stroke 6-36 months prior to inclusion and had at least one motor dysfunction. Patients were randomly assigned to "treated" or "cross" groups. Brain activity was assessed by SPECT imaging; neurologic functions were evaluated by NIHSS, ADL, and life quality. Patients in the treated group were evaluated twice: at baseline and after 40 HBOT sessions. Patients in the cross group were evaluated three times: at baseline, after a 2-month control period of no treatment, and after subsequent 2-months of 40 HBOT sessions. [31]
HBOT protocol: Two months of 40 sessions (5 days/week), 90 minutes each, 100% oxygen at 2 ATA. They found that the neurological functions and quality of life of all patients in both groups were significantly improved following the HBOT sessions while no improvement was found during the control period of the patients in the cross group. Results of SPECT imaging were well correlated with clinical improvement, and improvement of brain activity was detected mostly in regions of living cells (by CT) with low metabolic activity regions (detected by SPECT scan) or simply regions that show discrepancy between radiological anatomy and physiology. [31]
They concluded, HBOT can lead to significant neurological improvements in post stroke patients even at chronic late stages. The observed clinical improvements imply that neuroplasticity can still be activated long time after the onset of symptoms, in regions where there is a brain SPECT/CT (anatomy/physiology) mismatch. HBOT may have valuable therapeutic practice in other neurological disorders exhibiting discrepancy between the anatomical and functional evaluation of the brain. [31]
Chen Yu Chen et al., 2018, studied the hypothesis that HBOT both improves the clinical short-term outcomes and increases the number of circulating endothelial progenitor cells (EPCs) and antioxidant capacity in acute non cardio embolic stroke, The numbers of circulating EPCs [CD133+/CD34+ (%), KDR+/ CD34+ (%)], [26,30] biomarkers for oxidative stress (thiols and thiobarbituric acid-reactive substances), and clinical scores as National Institutes Of Health Stroke Scale (NIHSS), Barthel index (BI), and Modified Rankin scale (MRS) were prospectively evaluated in 25 patients with acute non-cardioembolic stroke under HBOT (pre-and post-HBOT), [29] The biomarkers and clinical scores were compared with those of 25 disease controls; they found that the numbers of KDR+/CD34+ (%) in the HBOT group following HBOT increased significantly, whereas the numbers of CD133+/CD34+ (%) also showed a tendency to increase without statistical significance. The mean high sensitivity C-reactive protein levels showed significant decrease post-HBOT follow-up in the HBOT group. Changes in KDR+/ CD34+EPC (%) numbers were positively correlated with changes in clinical outcomes scores (BI, NIHSS, and MRS) in the HBOT group, concluded that based on the results of this study, HBOT can both improve short-term clinical outcomes and increase the number of circulating EPCs in patients with acute non cardio embolic stroke.[29]
Since decade of research in HBOT with stroke, some studies have supported HBOT and others didn’t support, Carson S, et al 2005. Said that overall evidence is insufficient to determine the effectiveness of hyperbaric oxygen therapy in any subgroup of stroke patient and good quality studies are needed, [33] ALWaili NS, et al., 2005 announced “The results of HBO therapy in the treatment of patients with stroke are promising and warrant further investigation, .[26] and Efrati S, et al., 2013 said “The results indicate that HBOT can lead to significant neurological improvements post stroke patients even at chronic late stages”. [31]
Basic and clinical data suggested that HBO could be a safe and effective treatment option in the management of acute stroke, but further studies are needed to clarify its clinical utility when applied within the treatment window of 3–5 h (Sanchez 2013). The FDA has approved the use of HBO therapy for central retinal artery occlusion and HBO use for poststroke is currently off-label in the USA but Class 3 C recommendation of ECHM, expecting to be FDA approved in the future. [34]
In modern era of stroke thrombolysis and advanced neuroimaging, HBO may have renewed significance. If applied within the first few hours after stroke onset or in patients with imaging evidence of salvageable brain tissue, HBO could be used to lengthen the window for the administration of thrombolytic drugs. [34]
Finally, in the present study, cerebrovascular stroke patients received hyperbaric oxygen therapy in the first six months after the onset of the stroke, showed early higher improvement of physical and cognitive functions and overall quality of life. Further randomized double blind controlled studies are needed with larger sample size and more specification through inclusion of large sample of ischemic or hemorrhagic, thrombotic or embolic stroke and acute or chronic stage, alone or combined with other therapeutic modalities as thrombolytic therapy and stem cell therapy, with more specification of therapeutic HBOT dose for more enlightening of that era of stroke management. HBOT could be used safely in CVA patients with residual physical, cognitive function deficits and impairment normal quality of life in post stroke state.
To conclude, in this study we provide convincing results demonstrating that HBOT can induce significant neurological improvement in post stroke patients.
HBO therapy provides beneficial effects on ischemic and hemorrhagic brain injury and patients received HBOT showed earlier improvement of physical, cognitive functions and overall quality of life.
We recommend that hyperbaric oxygen therapy could be used in patients with cerebrovascular accident, as an adjuvant treatment to other modalities of treatment, in post stroke stage with residual physical, cognitive function deficits and impairment normal quality of life though more randomized controlled studies still needed.