Background: The coexistence of clearly demarcated glioma and vascular malformation is rarely identified. Astrocytomas represent the most common subtype found.
Methods: We conducted a systematic review of the literature published until December 2019, using the PubMed database, recording all histologically documented cases of AVM associated with primary brain tumor. We have reported for each case, study date, age, sex, intracranial site of the lesion, histopathological features, the neuroradiological techniques used for diagnosis, extension of surgical resection (biopsy, partial, total), adjuvant treatment (radiotherapy, chemotherapy, trans-arterial embolization) and overall survival.
Results: With our case, only 21 cases of angiographically detected arteriovenous malformation and histologically proven primary brain tumor, have been documented. The mean age was 42 years (range 8-72) with male prevalence (12 M, 9 F). Total tumor resection was obtained in 12 cases (57.1%), a partial resection in 4 cases (19.04%) whereas a biopsy was performed in 2 cases (9,5%); in 2 cases no information about the extent of resection was reported whereas in one 1 case no tissue sampling was obtained because of the death of the patient. AVM was treated with XRT in 5 cases (23.8%), subtotal resection (STR) was performed in 2 cases (9.5%), trans-catheter embolization was performed in 2 cases (9.5%) and in 1 case no treatment was performed for the patient's death.
Conclusion: The association between primary brain tumors and arteriovenous malformation is a rare entity that presents various pathogenic hypotheses and diagnosis can be difficult. In all these cases, we recommend a detailed histological examination of the parenchyma adjacent to the AVM.
Keywords: Xanthoastrocytoma, Arteriovenous malformation, Angioglioma, Hemorrhage, Glioma.
Abbreviations:
APXA = anaplastic pleomorphic xanthoastrocytoma
AVM = arteriovenous malformation
CT = computed tomography
DWI =diffusion weighted imaging
GTR =gross total resection
MCA = middle cerebral artery
MRI = magnetic resonance imaging
PXA = pleomorphic xanthoastrocytoma
RT = radiotherapy
STR = sub-total resection
TOF = time of flight
WHO = world health organization
The association between primary brain tumors and arteriovenous malformation (AVM) has been reported to reach 0.1% in the computed tomography (CT) scan era. Since the introduction of magnetic resonance imaging (MRI), the rate of association between these pathologies is supposed to significantly increase [1]. It includes ganglioneuromas, meningiomas, hemangioblastomas, neurilemmomas, craniopharyngiomas and gliomas. The latter represent the most common tumors detected in association with AVM and among gliomas, astrocytomas represent the most common subtype found [2]. Neovascularization, the formation of blood vessels de novo, and angiogenesis, the process of new vessel formation from pre-existing vasculature, are critical steps for the development of both AVMs andglial tumors. Within the latter, it is reasonable to speculate that local angiogenic factors could potentially contribute to the development of a vascular malformation [3]. Nazek et al. stated that a proliferation of oligodendroglial cells, in the cerebral tissue involved by the AVMs and in the adjoining white matter, does not represent a sufficient criterion for diagnosis of brain glioma. For this reason, some studies could probably have overestimated this aspect also due to the absence of recurrence in cases of subtotal resection [4].
Despite the skepticism, to date the international literature regarding angiographically documented intracranial AVM associated with histologically confirmed brain gliomas, diagnosed as combined lesions, consists of twenty cases and their existence as distinct entities is controversial. We present a literature review on association between primary brain tumors and AVM, adding our experience of a 46-year-old man undergoing surgical treatment for left parietal vascular malformation with histopathology suggesting co-present Anaplastic Pleomorphic Xanthoastrocytoma (APXA).
Clinical history and radiological findings
A 46-year-old man was admitted to the Emergency Department of the Policlinico Umberto I ° - “Sapienza” University of Rome, due to seizures without significant pathologies or history of head trauma. On neurological examination he appeared sleepy, collaborating, with ideomotor slowdown. No focal neurological deficits were detected. CT scan showed a spontaneous intraparenchymal hemorrhage in the left parietal lobe (Fig.1). To investigate the cause of the hemorrhage, a brain MRI confirmed the presence of a voluminous left parietal hemorrhage associated; Time of Flight (TOF) sequences showed an abnormal arterial vessel originating from the left middle cerebral artery (MCA) and bearing towards the hematoma. Cerebral angiography showed a left parietal AVM, size 3.5 cm, supplied by left MCA and draining in the posterior third of the superior sagittal sinus, Grade 2 according to the Spetzler-Martin classification (Fig.1). Considering clinical and radiological status, the patient underwent surgical removal of the AVM.
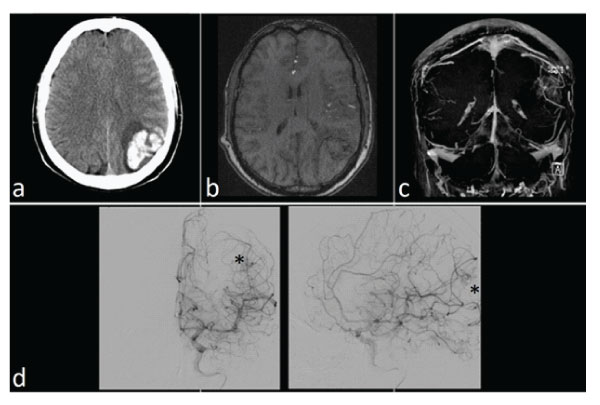
Figure 1: Preoperative radiological findings: a) CT scan showed the intraparenchymal hemorrhage in the left parietal lobe; b-c) MRI TOF and 3D angiographic reconstruction revealed the presence of an abnormal vascularization in the region of the hematoma; d) a cerebral angiography confirms the presence of the AVM (*).
Surgery
In prone position, using craniometric landmarks, a left parietal craniotomy has been performed. Once the dura has been opened, it has been possible to appreciate the presence of a subacute intraparenchymal hematoma. A preliminary intraoperative fluoroangiography has been performed confirming the presence of the AVM. Corticectomy has been performed in the site of the hematoma. Under microscopic guidance a lesion having the characteristic of the AVM has been detected. Arterial feeders have been accurately coagulated and cut. The nidus has been dissected from the brain parenchyma and the venous drainage has been consecutively closed. After removal of the nidus, intraoperative fluor-angiography has been performed confirming the complete resection of the AVM. The hemorrhagic clot has been completely removed.
Postoperative course
Postoperative angiography showed the disappearance of the pathological vascularization documented on preoperative investigations (Figure 2). Histopathological examination was suggestive for APXA (WHO grade III), with abnormal vascularization and BRAF V600E mutation (Figigure 3). A brain MRI with gadolinium was performed for this histological finding but no pathological enhancement was detected. Adjuvant chemotherapy with Temozolomide (according to Stupp’s schedule for 14 months) and radiotherapy were scheduled. Thirty-seven months later, for tumor recurrence, a new surgery was performed followed by adjuvant chemotherapy (according to Stupp’s program for 7 months). Unfortunately, a new neoplastic regrowth was detected 44 months after the first surgery but with involvement of the corpus callosum. The patient died for disease progression in corpus callosum and basal ganglia, with an overall survival of 47 months from diagnosis.
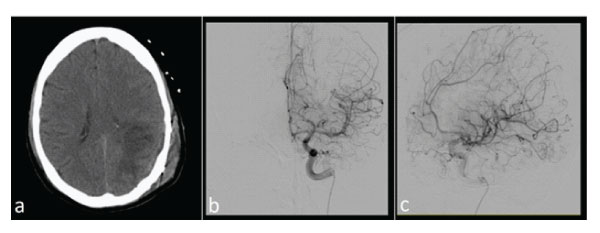
Figure 2: Postoperative CT scan (a) and angiography (b-c) showed the complete removal of the hematoma and the AVM.
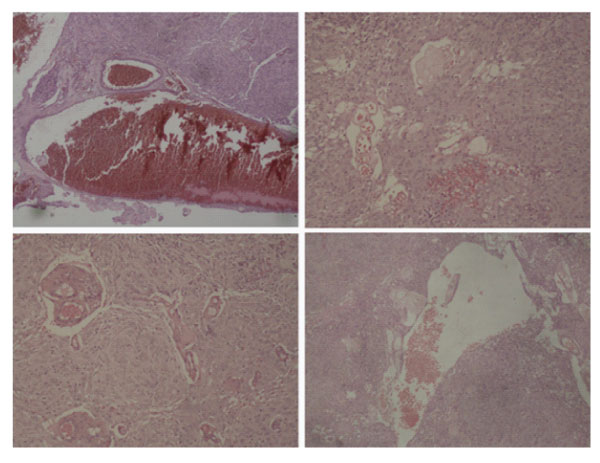
Figure 3: Microscopic examination documenting the presence of an hemorrhagic glial tumor with moderate cellularity. Cellular elements are characterized by big nucleoli and abundant eosinophilic cytoplasm and appears to be surrounded by fibrillar stroma. Some elements show pleomorphic nuclei with nuclear pseudocysts. Vascular mitosis, necrosis and proliferation are also present. All these aspects, coupled with the results of immunohistochemistry, suggested the diagnosis of anaplastic pleomorphic xanthoastrocytoma (grade III).
A systematic review of the literature has been performed using the scientific database PubMed by typing the following keywords: “xanthoastrocytoma and AVM”, “arteriovenous malformation and glioma”, “hemorrhagic brain tumor” and “angioglioma”. We only included articles written in English language. References of each article was analyzed to identify relevant manuscripts which were not identified through the initial research. We reported study date, age, sex, intracranial site of the lesion and histopathological features. In addition, the neuroradiological techniques used for diagnosis, extension of surgical resection (biopsy, partial, total), adjuvant treatment (radiotherapy, chemotherapy, trans-arterial embolization) and overall survival were also recorded.
The literature review through December 2019 revealed 20 publications for a total of 20 cases with a histological diagnosis of primary brain tumor in association with an AVM. Our case was added to those already described in the literature (Table 1). We also collected 8 publications for a total of 9 cases (including our case) of intracranial hemorrhage associated with histologically documented pleomorphic xanthastrocytoma (PXA) (Table 2).
Table 1: Cases reported of PXA with intracranial hemorrhage.
Case No |
Authors |
Age |
Sex |
Tumor site |
Hemorrhage |
Diagnosis |
Outcome |
1 |
Levy 1996 |
46 |
F |
Lt temporal |
ICH & SAH |
PXA |
Dead |
2 |
Yoshida 2005 |
61 |
F |
Lt temporal |
ITH |
PXA |
Survived |
3 |
Asano 2006 |
59 |
F |
Lt temporal |
ICH |
APXA |
Dead |
4 |
Lee 2007 |
64 |
M |
Lt frontal |
ICH |
PXA |
Survived |
5 |
Wind 2009 |
5 |
F |
Lt temporal |
ITH |
PXA |
Survived |
6 |
Yoshikawa 2010 |
60 |
F |
Lt temporal |
ICH & SAH |
PXA |
Survived |
7 |
Naganska 2013 |
36 |
F |
Rt occipital |
ICH |
PXA |
Survived |
8 |
Takamine 2019 |
11 |
F |
Rt temporal |
ICH |
PXA |
Survived |
9 |
Santoro 2021 |
46 |
M |
Lt parietal |
ICH |
APXA |
Dead |
ICH: Intra-cerebral Hemorrhage, ITH: Intra-tumoral Haemorrhage, Lt: left, Rt: right, SAH: Subar-achnoid Hemorrhage. |
|||||||
Table 2: Cases reported of radiological detected AVM and histologically proven primary brain tumor.
Case |
Authors |
Age |
Sex |
AVM/Tumor site |
Neuroimaging |
Treatment |
Tumor histology |
Outcome/ |
N° |
Follow-up |
|||||||
1 |
Fine 1960 |
15 |
M |
Parietal/Ventricular |
ventriculogram, angiogram |
TR, RT |
Oligodendroglioma |
NR |
2 |
Welcker 1966 |
25 |
F |
Parietal |
angiogram |
NR |
Astrocytoma |
NR |
3 |
Heffner 1971 |
17 |
M |
Subfrontal/Meningeal |
CT, angiogram |
PR (tumor), TR & RT (AVM) |
Low grade astrocytoma |
improved |
4 |
Crowell 1975 |
17 |
M |
Temporal |
CT,ventriculogram, angiogram (no shunting) |
PR |
Oligodendroglioma |
NR |
5 |
Zuccarello 1979 |
50 |
M |
Temporal |
angiogram |
NR |
Astrocytoma |
NR |
6 |
Ho 1981 |
63 |
F |
Thalamus/Cingulate |
NR, diagnosed at autopsy |
NR |
Astrocytoma |
NR |
7 |
Licata 1986 |
60 |
F |
Occipital/Temporal |
CT, angiogram |
PR (tumor), AVM not treated |
GBM |
Died, months 14 |
8 |
Martinez-Lage 1986 |
43 |
M |
Parietal/Ventricular |
CT, angiogram |
TR, RT |
Oligodendroglioma |
improved |
9 |
Goodkin 1990 |
9 |
F |
Parietotemporal |
CT, angiogram |
TR |
Anaplastic astrocytoma |
NR |
10 |
Malcolm 1991 |
41 |
M |
Frontal |
CT, angiogram |
TR, RT |
Astrocytoma |
Improved months 6 |
11 |
Lee 1996 |
45 |
M |
Temporo-occipital |
CT, angiogram |
TR |
PXA |
NR |
12 |
Harris 2000 |
57 |
M |
Temporo-occipital |
MRI, angiogram |
B, RT |
Anaplastic astrocytoma |
Died months 4 |
13 |
Ziyal et al., 2004 |
58 |
M |
Temporo-parietal |
MRI, postop angiogram |
TR (tumor), PR (AVM) |
high-grade glioma |
Improved |
14 |
McKinley 2008 |
55 |
F |
Parietal |
MRI, angiogram |
TR (tumor), TAE (AVM) |
Anaplastic olygodendroglioma |
Died day 6 |
15 |
Pallud 2009 |
35 |
M |
Frontal tumor, Rolandic AVM |
MRI |
TR (tumor) |
APXA |
Alive months 18 |
16 |
Aucourt 2012 |
65 |
M |
Frontal |
MRI, angiogram |
TR (tumor) |
GBM |
NR |
17 |
Soltanolkotabi 2012 |
8 |
F |
Thalamic |
MRI, angiogram |
TR (tumor), TAE (AVM) |
Pylocitic astrocytoma |
Alive |
18 |
Gmeiner 2013 |
72 |
F |
Temporal |
MRI |
TR, RT & ChT |
GBM |
Died months 12 |
19 |
Naganska 2013 |
36 |
F |
Occipital |
MRI |
TR |
PXA |
Alive |
20 |
Lohkamp 2016 |
71 |
F |
Thalamic |
MRI, angiogram |
B |
GBM |
Died months 6 |
21 |
Santoro 2021 |
46 |
M |
Parietal |
MRI, angiogram |
TR, RT & ChT |
APXA |
Died months 47 |
APXA: Anaplastic Pleomorphic Xanthoastrocytoma, B: Biopsy, ChT: Chemo-Therapy, CT: Computer Tomography, GBM: Glio-Blastoma Multiforme, MRI: Magnetic Resonance Imaging, NR: Not Reported, PR: Partial Resection, PXA: Pleomorphic Xanthoastrocytoma, RT: Radiation Therapy, TAE: Trans-Arterial Embolization, TR: Total Resection. |
||||||||
Among the cases of intracranial AVM associated with primary brain tumor, the mean age was 42 ± 29 years (range 8-72) with male prevalence (12 M, 9 F). Lesions were in parietal lobe in 5 cases (23.8%), in 4 cases (19.04%) in the frontal lobe, in 3 cases (14.2%) in the temporal lobe, in 1 case (4.7%) in the occipital lobe, in 3 cases (14.2%) at the thalamic level, in 3 cases (14.2%) the extension was temporo-occipital and in 2 cases (9.5%) temporoparietal.
In 7 cases (33.3%) a CT scan was associated with preoperative cerebral angiography, in 6 cases (28.5%) the angiogram was preceded by an MRI, in 4 cases (19.04%) surgical treatment was performed only after a brain MRI and in 3 cases (14.2%) only by intracranial angiography. In only one case (4.7%) in the pre-CT era a ventriculography was performed while in one case no images were acquired due to the patient's death.
Gross total tumor resection (GTR) was obtained in 12 cases (57,1%), a partial resection in 4 cases (19,04%) whereas a biopsy was performed in 2 cases (9,5%); in 2 cases no information about the extent of resection was reported whereas in one 1 case no tissue sampling was obtained for the patient’s death.
AVM was treated with radiotherapy (RT) in 5 cases (23.8%), subtotal resection (STR) was performed in 2 cases (9.5%), transarterial embolization was performed in 2 cases (9, 5%), in 1 case no treatment was performed for the patient's death and in 9 cases the data was not reported.
Primary brain tumor was Glioblastoma Multiforme in 5 cases (23.8%), astrocytoma in 8 cases (38%) including in 2 cases (9.5%) anaplastic astrocytoma and in 1 case (4.7%) pilocytic astrocytoma; in 4 cases (19.04%) the histopathological examination was indicative of oligodendroglioma including 1 case of anaplastic oligodendroglioma. One PXA was found in 4 cases (19.04%) including 2 cases of anaplastic type. Among the 18 cases (85,7%) surgically treated for brain tumor, adjuvant treatment was used in 2 cases (11%) combining chemo and radiotherapy. Follow-up was reported in 8 cases with a mean survival of 13.4 months.
Among the 9 cases of cerebral haemorrage associated to PXA, mean age was (range 5-64), with female prevalence (2 M, 7 F). In 6 cases (66%) tumor and haemorrage were in the temporal lobe, in 1 case (11%) in frontal lobe, in 1 case in the occipital lobe and in 1 case in the parietal lobe. Histology was PXA in 7 cases (77%) and APXA in 2 cases (22%). In 2 cases (22%) subarachnoid heamorage was associated with intraparenchymal one. In 3 cases (33%) patients died for the heamorragic event.
PXA (WHO grade II) is an astrocytic tumor that grossly appears as a superficial solid and cystic mass with involvement of the leptomeninges, moderate cellularity, predominantly pleomorphic with foci of lymphoplasmacytic infiltration without any necrosis and rare mitoses [5,6,7]. Pleomorphic Xanthoastrocytoma (PXA), term firstly coined by Kepes et al in 1979, is a rare, circumscribed glioma composing <1% of all astrocytic tumors. It is mostly diagnosed in the second decade of life with mean age at diagnosis is 29 ± 6 years with no gender predilection [5,6,7]. Initial presenting symptoms include seizures, headaches, nausea, vomiting, diplopia and somnolence [8]. According to the World Health Organization (WHO) classification, since 2016, two different entities have been pointed out, based on histopathologic features: grade II PXA and grade III anaplastic PXA. In 1983 Weldon-Linne described the first case of recurrent PXA with malignant transformation (WHO grade III) [9]. Anaplastic Pleomorphic Xanthoastrocytoma composes 1550% of all PXA; it has magnetic MRI features like those of other high-grade astrocytomas, namely more heterogeneous contrast enhancement, peritumoral edema, lower rADCmin ratio on DWI, and higher rCBVmax ratio on DSC-PWI [10]. The enhancement pattern of APXA could be explained by the necrosis, hemorrhage, and vascular proliferation within the tumor, which was observed by Rutkowski [11]. On histological examination it is defined by the presence of necrosis associated with high mitotic activity, usually> 5 mitosis / 10 high-power fields and increased cellularity [12]. Like our case, one of the main molecular characteristics is BRAF V600 gene mutation that have the highest oncogenic potential [13,14]. This mutation divides PXAs into two clinically relevant subgroups for natural history and response to therapy: BRAF-mutated PXA shows superior survival compared to wild type BRAF and exhibits responses to BRAF-targeted therapies [15,16]. Total resection should be the surgeon’s goal, correlating with 10-year overall survival of 82% for PXA [17]. Otherwise, APXA has a 5-year OS rate of 57% [11]. A meta-analysis of 167 patients with grade II PXA does not demonstrate an association between adjuvant therapy with better oncological outcomes while radiotherapy is useful for residual or recurrent tumor [18]. Generally traditional chemotherapy has been considered minimally effective or ineffective for the treatment of PXA [19], while in progressive disease or APXA, when surgery or radiotherapy is not always a viable option, systemic treatments are often employed [8].
The incidence of hemorrhage in gliomas is approximately 1.42.6% and its frequency increases with the WHO classification [1]. It is more common in high-grade gliomas such as glioblastomas and anaplastic astrocytomas, which show microvascular proliferation and necrosis. Reticular type capillaries are sometimes seen in lower grade gliomas and have been found associated with hemorrhage [20,21].
Intracranial hemorrhage associated with PXA is rare and to date nine cases have been reported in the literature, including our experience (Table 2). The lesions, consisting of mixed tumors of glial and vascular origin, particularly cavernous or arteriovenous type, have often been referred to as angiogliomas. The term “angioglioma” was coined by Councima et al. to describe a cerebellar vascular neoplasm with characteristics of what is now recognized as the cellular variant of hemangioblastoma and therefore appears to be valid for rare cases of low-grade glioma with a distinct vascular pathology. Angiogliomas should not be confused with high-grade neoplastic gliomas, which due to significant neovascularization, can be misdiagnosed with AVM [2].
Pathogenetic suggestions consider these angiogliomatous lesions as a reactive astroglial neoplastic proliferation secondary to a pre-existing vascular malformation and/or haemorrhages [1,22]. Four cases have been described in the literature, showing the initial appearance of the AVM and the subsequent finding of the tumor within it; histological examination showed hemosiderinladen macrophages and reactive gliosis: it could represent the transition between the vascular precursor and glioma. The possibility that an anaplastic glioma could induce de-novo vascular malformation or that a bleeding vascular malformation could induce secondary neoplastic changes in perivascular glial tissue is still under discussion [1,23,24]. More studies are needed to provide an adequate answer.
The coexistence of clearly demarcated glioma and vascular malformation is rarely identified. With our case, only 21 cases of angiographically detected AVM and histologically proven primary brain tumor, have been documented (Table 1). AVM might be associated with different glial components including low- and high-grade astrocytoma, oligodendroglioma, mixed oligo-astrocytoma or glioblastoma. We have reported the case of a lesion with two separate components of APXA and AVM, which form a common tumor mass. Although the angiographic and intraoperative appearance of the lesion had the characteristics of an AVM, the definitive histopathological diagnosis revealed an APXA. Only three previous cases of this association have been reported: two cases with WHO grade III and one case WHO grade II (Table 1).
The association between primary brain tumors and arteriovenous malformation is a rare entity that presents various pathogenic hypotheses and diagnosis can be difficult. This possibility could be overestimated as reported in the literature. However, in cases of AVM, we believe that a detailed histological examination of the adjacent brain parenchyma and adequate clinical and radiological follow-up are necessary to avoid misdiagnosis, in the possibility of coexistence of a PXA / APXA or other forms of underlying primary brain tumor.