Previously the opinion has been formed about significant relationship between stress and various diseases including cardiovascular disorders. However, the advances in biomedical science during the last decades provoke the necessity of updating this opinion. Therefore, this work aimed at presentation of bibliographic data that confirm the important role of stress in the etiopathogeny of arterial hypertension and other cardiovascular diseases. In addition, major attention was focused on the role of hormonal stress mediators and principally, glucocorticoids in pathogeny of cardiovascular disorders. Finally, the evidence was described for developing etiopathogenic mechanisms of hypertension and other cardiovascular diseases, on the basis of stress and its mediators, along the whole scale of pre- and postnatal ontogeny and till the senescence, thus confirming the importance of ontopathogenic model as one of possible theoretical axes of biomedicine, in the framework of DOHaD – developmental origins of health and disease.
Keywords: Cardiovascular Disorders; Glucocorticoids; Ontopathogeny; Stress.
Abbreviations: GC: glucocorticoids; CV: cardiovascular
Previously an essential role was described for stress and its hormonal mediators, glucocorticoids (GC) and catecholamines in the etiopathogeny of neuropsychiatric [1] and metabolic disorders [2]. However, at present the main mortality is caused by cardiovascular (CV) diseases, what augments the necessity of describing the role of stress and its mediators in the etiopathogeny of such diseases. Moreover, among the CV disorders, systemic arterial hypertension is saliently important as highly prevalent disorder and one of the principal risk factors for ischemic heart disease and stroke, as well as heart or kidney insufficiency [3], thus explaining our intention to devote more space in this article for hypertension.
First of all, we should outline that this topic was a priority already for the founder of stress concept, Hans Selye [4]. After half a century of research, various authors continue to pay their attention on the importance of stress for the pathogeny of ischemic heart disease and acute myocardial infarction, stroke and systemic arterial hypertension [5,6,7], focusing in part on the role of catecholamines, adrenaline and noradrenaline, as well as some other pressogenic agents: angiotensin II, argininevasopressin and endothelin [8,9,10].
However, our principal attention will be payed to GC, since these hormones are used as important drugs with antiinflammatory and immunosuppressive action in many areas of modern medicine. In fact, higher levels of endogenous GC in biological fluids (saliva, blood components etc.) serve as important indicators for the formation of atheroma plaques [11,12] and calcification process in arterial blood vessels [13,14]. Moreover, GC potentiate vasoconstrictor actions of catecholamines, angiotensin II and endothelin and on the other hand, inhibit the production of vasodilating substances, nitric oxide and prostacyclin [15,16]. Unfortunately, prolonged use of GC in elevated doses in rheumatology, for the treatment of bronchial asthma and in patients with organ transplants is capable to provoke higher mortality, including that in CV disorders [17,18].
Various authors outline that pathogenic influence of GC in excess is pleiotropic, since their exaggerated use results in higher risk of several components of metabolic syndrome: insulin resistance and type 2 diabetes mellitus, systemic arterial hypertension and blood hypercoagulation [19,20]. On the other hand, GC are saliently important in the etiopathogeny of depression and some other neuropsychiatric disorders [1]. Meanwhile, the depression serves as important risk factor for CV diseases and also as disorder that aggravates a situation after acute myocardial infarction, increasing the mortality or at least, causing enormous difficulties for rehabilitation [21,22]. Finally, the depression and stress appear to have similar pathophysiologic mechanisms [23].
It is important to note that stress has significant contribution for CV diseases including hypertension in occupational medicine [24]. On the other hand, stress management has principal interest in CV rehabilitation, since one of the most important factors of mortality in depressed patients is CV [25]. As a matter of fact, in modern world, full of demands, expiry dates and other frustrating complications, many persons are exposed to stress, including the work circumstances and problems, especially in the intermediate age categories (35-54 yr) [26]. In addition, in postmenopausal period (approx. after the age of 50 yr) the women lose their antihypertensive estrogenic protection, probably based on stimulated production of vasodilatory agents [16].
The role of glucocorticoids in the ontopathogeny of cardiovascular disorders
The importance of GC is especially significant as the targets and mediators of the phenomena of programming / imprinting, in the paradigm of developmental origins of health and disease (DOHaD) [27]. This paradigm was elaborated primarily in a series of epidemiologic studies 20-30 yr ago and soon after that, in the investigations using experimental models of laboratory animals [28]. In particular, experimental models have clearly shown that administration of exogenous GC to laboratory animals (principally, rats and sheep) during gestation resulted, as referred to their offspring, in hypertension that was amplified along postnatal ontogeny scale [29,30]. Obviously, the necessity emerged soon to explain this pathogenic capability of GC and paradoxically, the principal organs important for such explanation were the kidneys and not those of CV system [31] (see Fig. A).
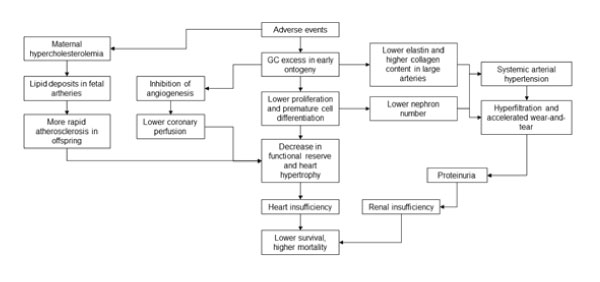
Figure A: Possible mechanisms of glucocorticoid involvement in the phenomena of programming / imprinting.
In fact, the use of exogenous GC at the end of gestation and the beginning of postnatal life caused a diminution of the number of nephrons, principal morpho-functional units of kidneys. It appears that partially such pathogenic GC action is related to their capacity of accelerating the maturation of various tissues during the transition from pre- to postnatal life, with the diminution of cell proliferation and increase in the degree of cellular differentiation [32,33,34].
But the mechanisms that are much more important in this pathophysiologic sequence of events, appear to include the consequences of such diminution of nephrons number in postnatal life till the senescence. Here it is worth to remember at first that the kidneys, as well as other important organs such as heart, are highly complex and do not show, in significant mode, the capacity of regeneration, especially in adult life. Therefore, in order to meet the demands of growing body, the nephrons resting in lower number, suffer a certain hypertrophy, however their hyperfiltration, along the time scale, provokes accelerated wearand-tear, revealed in the augment of blood pressure, proteinuria and more rapid loss of kidneys functional ability, arriving finally at renal insufficiency [35,36]. Therefore, it is not a surprise that the resultant outcome of this pathophysiologic sequence of events is a significant diminution of a personal survival, principally in the intermediate age categories and in senescence [37].
Obviously, this pathophysiologic mechanism is important, first of all, for hypertension, however there exist another explanations that describe how GC augment the risk of various disorders, in this case via the alterations in proper organs of CV system (see also Fig. A). In experimental models it was shown that neonatal GC administration decreased cellular proliferation in the heart, provoking in this way the diminution of functional reserve and higher tendency to heart hypertrophy in posterior life [38, 39]. Meanwhile, the hypertrophy, especially of left ventricle, serves as important risk factor for heart insufficiency in posterior life [40].
In addition, GC excess in early ontogeny caused a disequilibrium between cardiomyocyte growth and the number of accompanying capillaries, due to the capacity of these hormones to inhibit angiogenesis [41,42]. As a result of such disequilibrium, a predisposition is formed to lower coronary perfusion, what can have a critical role in the situations of functional overload of the heart during some stress situations.
On the other hand, it was suggested that adverse environment in perinatal period is able to interfere with biosynthesis of elastin, an important protein of large arteries that is responsible for their elasticity and therefore, a continuous flux of blood during diastole (see again Fig.A). It is worth to mention that elastin is not produced in postnatal life, but this does not affect a personal survival immediately, due to extremely long half-life of this protein, more than 40 yr. However, along the time scale, during several decades and due to constant mechanical stress, the elastic capacity of arterial wall begins to decrease slowly, what partially explains almost universal augment of blood pressure with the age. The problem here is that in the case of lower elastin content, due to the influence of adverse environment, including GC excess in perinatal ontogeny, the period of CV system functioning without failures may really diminish [43,44].
Clearly enough, arterial blood vessels possess a compensatory mechanism, i.e collagen synthesis, but unfortunately, such mechanism is accompanied by the augment of vascular wall width, and moreover, it does not re-establish the necessary elasticity, thus allowing for greater vulnerability of large arteries, in the face of mechanical stress [45].
Finally, maternal hypercholesterolemia during gestation, including the sequelae of hypercortisolism, can provoke an augment of lipid deposits in the fetal arteries and therefore, more rapid progression to atherosclerosis in the offspring [46].
Ontopathogenic model in relation to pediatric use of glucocorticoids
Obviously, the first emergent question is why GC are widely used in medicine as a whole and in pediatrics, if their adverse effects are so salient, including in long term? In this regard, it is worth to remember briefly a history of these drugs. After their discovery at the end of the decade of 1950s, GC presented themselves at first in almost perfect fashion, with excellent efficacy for the treatment of rheumatoid arthritis and many other inflammatory and autoimmune disorders [47]. On the other hand, from the end of the last century, GC use was promoted in obstetrics and neonatology, by means of their administration to pregnant women or premature infants, principally in order to accelerate pulmonary maturation in perinatal period [48]. In many cases GC literally save the lives and unfortunately, till the present moment nothing better exists on pharmaceutical market for their substitution. Therefore, as several authors affirm [49], some physicians make a ”Faustian agreement”, considering simply that the benefits are more important, than the risks… Certainly, pharmaceutical industries are looking for the ways to alleviate adverse GC effects, developing, e.g. selective agonists of GC receptors (SEGRA), but unfortunately, in practice the latter drugs have not exited from pre-commercial phase.
Final comments
As a matter of fact, chronic stress is able to provoke elevated and intense exposure to endogenous GC in such a way that can augment a predisposition to age-related disorders and to the proper aging, including in long term, if such exposure occurs already during early ontogeny, pre- and postnatal [50]. This is especially important in the cases of infantile and juvenile work in excess, since chronic stress of such work and adverse conditions in general serve in cumulative mode for the phenomenon of biological embedding in postnatal life, thus augmenting the complexity of the ontopathogenic model [51].
Therefore, previously we have performed a search in world literature for the ways of diminishing the adverse impact of exaggerated stress or GC in excess. It was shown that various hormones and some non-hormonal substances are able to diminish unfavourable GC effects both in perinatal period and in adult life. Among them one can mention melatonin and neuroactive steroids [52], somatolactogens and related peptides [53,54], as well as some antioxidants [55]. The principal problem is that for their ample use in combination with GC, a number of prolonged investigations and large-scale clinical trials are necessary. Therefore, on our opinion, it is worth to pay more attention at present to non-drug options of antistress procedures [56], but this topic would be important part of our future publication.
Notes added in proof
In fact, we study GC and stress, both in experiments and theoretically, for already 30 years and nevertheless, a lot of questions remain poorly understood yet, despite of enormous efforts of scientific community since the discovery of these hormones close to the end of the first half of 20th century. One of the causes of such situation is the paradigm of translational biomedicine, when the data obtained on experimental models of laboratory animals (rats, mice and others) are transferred for interpretation in humans, due to intrinsic differences between species. However, earlier and at present we don’t have any other choice, principally because of ethical problems and logistics of experimental studies. For example, bioethical aspects are especially complicated, when the data should be obtained on pregnant women and newborn infants, and at least in these cases experimental models on laboratory animals are the only solution. Moreover, because of much shorter lifespan and duration of ontogenic periods, rodents are the unique option for rather expensive experimentation related to ontopathogenic model. Even with such advantages and excuses, the problems still remain, including the degree of maturity at birth and type of placenta, but sometimes, as in our case, at least partially, these differences can even help, considering that neonatal rats are similar to premature human infants.
What for GC, not these hormones per se are problematic, but their use in excess and paradoxically, also the lack of them in situations like post-adrenalectomy, because of enhanced levels of pro-inflammatory cytokines. On the other hand, till the present moment it is not completely clear, where is the threshold separating physiological and pharmacotoxicological GC effects. Really, GC possess biphasic action describing the phenomenon of hormesis, but in the case of long-term treatment with them, allostatic load and even overload can emerge (see discussion in [57]), since not only GC dosage, but also the time period of treatment are important, generating the product of dosage (in mg) multiplicated by the time of exposure (e.g., in days). Moreover, the regimen of GC pharmacotherapy (oral or inhaled, every day or on alternate days) and the pharmaceutical GC forms with different potency and lipid solubility are also highly important [47].
Therefore, we would like to address more dedicated readers to references cited, as well as to our previous papers, considering also that many of them were published in the Open Access journals and in addition, they are mirrored on ResearchGate and Academia websites. In conclusion, our goal continues to be not acting in favour of steroid phobia, but only for alerting clinical practitioners and other health professionals (chiefly, specialists in nursing and pharmacy) about the necessity of highly skillful management of GC pharmacotherapy, especially in perinatal period and in childhood.
Early version of this work was presented in abstract form at 20. Congresso de Stress da ISMA-BR, Porto Alegre - RS, Brazil on June 2020 (in Portuguese).
Disclosure
The author declares there is no conflict of interest.
Funding
This study was not supported by any funding.
Acknowledgement
Our great thanks are to anonymous reviewer for important proposals that allowed to significantly improve this article.