Targets for LDL-cholesterol (LDL-C) that should be aimed at have been defined by international societies. In high-risk patients they are set at 1.8 (70 mg/dL) and 1.4 mmol/L (55 mg/dL). We report our experience with respect to the use of statin and PCSK9 inhibitors in patients with familial hypercholesterolemia (FH). It was observed that in 37.5% of FH patients the target LDL-C level, corresponding to the risk category, was not achieved. The reasons for this failure, like low adherence, high initial lipid profiles, and medical errors in prescribing combination therapy, are discussed. In the future, it will be necessary to improve this situation or to add another therapeutic option, like for instance a lipoprotein apheresis (LA). In the second part of this paper, data measured in LA patients are given. Though the lipid-lowering therapy was optimized including PCSK9 inhibitors, the LDL-C target of 1.0 mmol/L, which was recommended in patients who suffered from at least two cardiovascular events in the last two years, was reached only in a small portion, when calculating interval mean values. Nevertheless, patients who did not show such low LDL-C levels did not all of them develop cardiovascular events. Thus, the LDL-C target levels should be more individualized. The extracorporeal therapy with LA provided excellent acute reductions of Lipoprotein(a), but when looking at interval mean values, “normal” values (< 75 nmol/L) were seen only in very few patients. Inhibitors of (Lp(a)) synthesis, like Pelacarsen, probably will change this situation.
Keywords: Familial Hypercholesterolemia; Statins; PCSK9 Inhibitors; LDL-Cholesterol; Lipoprotein(a); Lipoprotein Apheresis
The atherogenic risk is clearly increasing with increasing concentrations of low-density lipoprotein cholesterol (LDL-C) and of Lipoprotein(a) (Lp(a)). In order to prevent a progress of atherosclerotic lesions or of cardiovascular events (CVE) for both parameters the slogan is “The lower, the better”[1,2,3].
In the last years, the recommended target level for LDL-C has been fixed for high-risk patients at <1.8 mmol/L (<70 mg/ dL) and < 1.4 mmol/L (55 mg/dL), respectively [4,5] In addition, a reduction of LDL-C by at least 50 % from the initial value should be aimed at. For very high-risk patients with two or more events in the last two years, an even lower LDL-C (< 1.0 mmol/L; < 40 mg/dL) has been suggested.
For Lp(a) no officially accepted target concentration was proposed. The general feeling is that a level below 120 nmol/L (equivalent to about 50 mg/dL) should be reached. But studies clearly show that Lp(a) concentrations higher than 75 nmol/L (equivalent to 30 mg/dL) may be already atherogenic[6,7].
It is quite clear that in patients with a genetically determined lipid disorder these target values can only be obtained by the application of lipid-lowering drugs and – in patients with extremely high atherogenic risk – by lipoprotein apheresis (LA). Though all patients should get a dietary advice, an improvement in eating habits is usually not enough. Moreover, Lp(a) concentrations cannot be influenced by nutrition or physical activity.
For reducing LDL-C concentrations, several drugs can be prescribed: statins, ezetimibe, bile acid sequestrants, bempedoic acid, monoclonal antibodies against PCSK9 (Evolocumab, Alirocumab), small interfering RNA (siRNA) against PCSK9 (Inclisiran). The indication for the extracorporeal LA is usually given only in patients who showed CVE despite being treated with lipid-lowering drugs when tolerated.
This paper reports the experience of the authors with respect to reaching the recommended target levels in two groups of patients:
hypercholesterolemia or an elevation of Lp(a) (and usually a progress of their atherosclerosis which was documented by new CVE or by imaging technique).
FH is the most common genetic metabolic disorder when Total cholesterol (TC) and LDL-C increase from childhood. European and U.S. guidelines recommend identifying subjects with FH in order to start LDL-C–lowering therapy early in life to prevent ischemic heart disease (IHD) and premature death [4,5,8,9]. FH patients belong to the categories of high and very high risk. Accordingly, the target LDL-C levels according to the latest recommendations should be 1.8 (70 mg/dL) and 1.4 mmol/L (55 mg/dL), respectively.
In a previous paper we have shown that the achievement of a target LDL-C level less than 1.8 mmol/L (70 mg/dL) in FH patients was 22.6 %, and only 5.7 % patients achieved the target level less than 1.4 mmol/L (55 mg/dL) on statin therapy [11].
We evaluated the use of statins in 191 patients with FH (75 men (39.3%)). 124 patients (64.9%) took the drugs, 67 patients (35.1%) did not use them. High doses (HD) of statins received 31 patients of the total group, which accounted for 25% of patients treated with statins, 25 persons (20.1 % among the treated with statins) have received average doses of statins, lowdose statin received 68 (54.8 % among those on statins) [11]. Clinical characteristics of FH patients receiving HD of statins and hypolipidemic effectiveness of HD statins are presented in Table 1.
Table 1: Clinical characteristics of FH patients receiving high doses (HD) of statins or different statin doses.
Indicator |
HD statins |
Patients with different statin doses |
Total amount of patients |
Total, n (%) |
31(16.2%) |
124 (64.9%) |
191 |
Males, n (%) |
9 (29.0%) |
44 (35.5%) |
75 (39.3%) |
Achievement of LDL-C target levels, n (%) |
6 (19.4%) |
18 (14.5%) |
18 (9.4%) |
Reduction of the initial level of LDL-C by ≥ 50%, n (%) |
20 (64.5%) |
64 (51.6%) |
64 (33.5%) |
Nonstable IHD |
0 (0,0) |
12 (9,7%) |
15 (7.9%) |
Adverse events, n (%) |
4 (12.9%) |
14 (11.3%) |
15 (7.9%) |
Adherence, n (%) |
24 (77.4%) |
86 (69.4%) |
86 (45.0%) |
Duration of therapy (years) |
5.3 ± 0.7 |
3.8 ± 0.8 |
3.7 ± 0.5 |
Initial Lipid levels |
|||
TC initial, mmol/L |
10.2 ± 0.3 |
9.7 ± 0.3 |
9.4 ±0.1 |
LDL-C initial., mmol/L |
7.4 ± 0.3 |
7.0 ± 0.2 |
6.7 ± 0.1 |
TG initial, mmol/L |
1.9 ± 0.1 |
2.0 ± 02 |
1,8 ± 0,1 |
HDL-C initial, mmol/L |
1.5 ± 0.1 |
1.5 ± 0.1 |
1.5 ± 0.03 |
Lipids during treatment |
|||
TC treated, mmol/L |
5.4 ± 0.2 |
5.7 ± 0.2 |
6.2 ± 0.2 |
LDL-C treated, mmol/L |
3.3 ± 0.3 |
3.5 ± 0.2 |
3.9 ± 0.2 |
TG treated, mmol/L |
1.9 ± 0.3 |
2.1 ± 0.2 |
2.2 ± 0.2 |
HDL-C treated, mmol/L |
1.3 ± 0.1 |
1.4 ± 0.07 |
1.4 ± 0.06 |
IHD: ischemic heart disease; TC: Total cholesterol; TG: Triglycerides |
|||
Statins reduced LDL-C up to 50% in our patients with heterozygous FH (HeFH). We have analyzed the main reasons for the initial refusal to take statins in patients with FH. In 87% it was the fear of patients developing adverse effects of drugs. An important limiting factor to using HD statins was statin intolerance. Myalgia was observed in 12%, that was the main reason for decreasing the doses or discontinuing the statins. An increase of transaminases was seen in 35%, skin rashes - in 12%. The percentage of patients, who independently refused to take statins, was 29% [6].
In patients receiving a combination therapy with PCSK9 inhibitors (iPCSK9) goal levels were achieved in 62.5%. Thus, the application of iPCSK9 in FH patients improves the achievement of target levels by 40%.
We established that adherence to statin therapy was 3.5 ± 0.3 balls by Morisky- Green [12], but adherence to iPCSK9 was higher, 3.99±0.01 balls (p<0.05), and it did not depend on presence of cardiovascular disease and age.
Table 2: Dynamics of laboratory parameters on the standard lipid-lowering therapy and after PCSK9 inhibitors (iPCSK9).
Time frame |
TC, mmol/L |
LDL-C., mmol/L |
HDL-C., mmol/L |
TG, mmol/L |
Lp(a), g/L |
Creatinin, mcmol/L |
ALT, ME/L |
AST, ME/L |
Glucose, mcmol/L |
Initially (n=72) |
10.2± 0.23 |
7.3±0.16 |
1.46±0.1 |
1.82±0.3 |
0.48±0.06 |
79.8±3.4 |
35±1.3 |
36.1±2.3 |
5.3±0.6 |
Hypolipidemic therapy before iPCSK9 (n=72) |
5.8±0.3 |
3.9±0.3 |
1.3±0.2 |
1.7±0.4 |
- |
80.3±4.0 |
34±1.2 |
34.2±1.5 |
5.2±0.5 |
3 months on iPCSK9 (n=64) |
3.1±0.02 |
1.7±0.02 |
1.21±0.3 |
1.6±0.4 |
0.34±0.03 |
78.5±4.3 |
34.1±0.8 |
35.4±2.8 |
4.96±0.8 |
6 months on iPCSK9 (n=70) |
3.9±0.04 |
1.68±0.03 |
1.3±0.2 |
1.65±0.2 |
0.33±0.04 |
77.6±3.3 |
34.2±0.8 |
36.5±3.2 |
5.0±0.6 |
1 year on iPCSK9 (n=72) |
3.98±0.07 |
1.75±0.02 |
1.29±0.3 |
1.7±0.6 |
0.34±0.03 |
79.2±5.8 |
35.4±2.3 |
35.7±3.6 |
5.1±0.4 |
1.5 years on iPCSk9 (n=64) |
4,0±0.03 |
1.78±0.04 |
1.3±0.4 |
1.68±0.5 |
0.33±0.02 |
78.6±6.7 |
34.8±1.7 |
35.7±3.7 |
5.1±0.3 |
2 years on iPCSK9 (n=69) |
3.99±0.04 |
1.76±0.03 |
1.32±0.3 |
1.67±0.4 |
0.33±0.04 |
78.4±4.5 |
35.3±1.4 |
34.5±4.3 |
4.9±0.4 |
On iPCSK9 therapy the mean level of LDL-C decreased by 56.4% after 3 months (initial level before iPCSK9 on another hypolipidemic therapy was 3.9 ± 0.3 mmol/l, and after 3 months therapy with iPCSK9 it was 1.7 ± 0.2 mmol/L). The hypolipidemic effect did not change during the follow-up. Significant differences between the indicators of TC and LDL-C were revealed in the subgroups of «initially» and «hypolipidemic therapy before iPCSK9» for both indicators (p<0.001); and also between groups «hypolipidemic therapy before iPCSK9» and the group «three months on iPCSK9» for both indicators –TC and LDL-C (p<0.001). There were no significant differences between other indicators of the lipid spectrum and the presented biochemical indicators. With the continuation of therapy with iPCSK9, the hypolipidemic effect (TC, LDL-C levels) remained constant. There were no significant differences between TC and LDL-C during the continuation of therapy (p>0.05).
The target levels for LDL –C on iPCSK9 therapy were achieved in 62.5% of FH patients. In other words, in 37.5% of FH patients the target LDL-C level, corresponding to the risk category, was not achieved. The reason for this was objective difficulties with hypolipidemic therapy. At first, the initial TC and LDL-C concentrations were very high, so it was very hard to achieve target LDL-C level less than 1.4 mmol/L. However, in two cases, patients had achieved LDL-C concentration of 1.7 mmol/L, it was not a bad result, and we had achieved the target level, which was recommended in the previous version of the consensus document for this patient category. Besides, in three patients we performed a monotherapy with iPCSK9 because of an intolerance to other hypolipidemic therapy. In 93% patients, we achieved a decrease of LDL-C concentrations by 50% from initial values. And in 7% (in two cases) of patients the degree of decrease was less, both patients had high level of Lp(a) and one of them has low glomerular filtration rate, as a possible reason of the iPCSK9 low reaction.
The Lp(a) levels in patients with definite FH on iPCSK9 therapy decreased by 30% from initially level (from 0.48 ± 0.06 g/L to 0.34 ± 0.03 g/L) after three months iPCSK9 therapy (Table 2).
We also analyzed the main mistakes in iPCSK9 therapy which were made by practitioners (some aspects were observed in some patients at the same time). They are the following: 1) ignorance of drug doses – 35%; 2) ignorance of the frequency of administration, some doctors used the drug one time a week instead of using it one time in two weeks, some doctors use the drug once a month instead of using it once every two weeks 25%; 3) doctors considered the drug extremely strong and were afraid to prescribe it for more than one month without control – 30%; 4) temporary therapy – 25%; 5) discontinuation of therapy when the target levels of LDL-C were reached or the level of LDL-C was less than 1 mmol/L – 15%; 6) withdrawal of drugs during treatment for COVID-19 and in the post-covid period – 40%; 7) non-compliance with the rules of transportation and storage (cold chain) – 10%; 8) the use of insufficient doses of statins as part of combination therapy – 35%; 9) fear of using multicomponent lipid-lowering therapy, and as a result, one of the components was excluded from therapy, for example ezetimibe – 15%.
In total, in 37.5% of FH patients the target LDL-C level, corresponding to the risk category, was not achieved. So that patients are needing a fourth component of hypolipidemic therapy, like for instance an apheresis therapy. In our data, the main reasons of insufficient effectiveness in achieving the target levels of LDL-C by using three-component therapy, including iPCSK9, were the reduced hypolipidemic efficacy of iPCSK9 (7%), doctors mistakes in administration of hypolipidemic therapy combination (30%), reasons associated with patients (inability/ unwillingness to take HD of statins, low adherence to lipidlowering therapy, fear/unwillingness to use multicomponent lipid-lowering therapy, 33%); and initially high indicators of the lipid spectrum (30%).
We published a study in LA patients comparing those who developed CVE with those who did not[13]. We used 6 different LA methods, the observation time during the extracorporeal therapy was about 5 years in the mean (range1.25 to 26.24 years).As a rule, most patients underwent LA therapy weekly.
As a whole 108 patients were included. They were divided into 2 groups: 1. CVEx/0 (that means CVE before the start of the LA therapy, no CVE during LA therapy) – 60 patients (42 males, 18 females), mean age at the first LA session 55 years (range 29 – 75 years), 2. CVEx/1+ (that means CVE before the start of the LA therapy, at least on CVE during LA therapy) – 48 patients (28 males, 20 females), mean age at the first LA session 60 years (range 41 – 75 years). The analysis showed two significant differences between these two groups: 1. CVEx/1+ patients were significantly older, 2. CVEx/1+ patients had suffered from a higher number of CVE before the start of LA treatment (median 3 (IQR 2 – 5) versus median 1.5 (IQR 1 – 3) in the CVEs/0 group). Moreover, the number of CVE before LA therapy correlated positively with the number of CVE during the extracorporeal treatment in the CVEx/1+ group (correlation coefficient 0.331, p 0.022). The major conclusions of these data were that an LA initiation should be performed earlier and after a lower number of CVE.
The gender distribution and the duration of the LA therapy were not different. With respect to lipid levels (LDL-C, Lp(a), triglycerides (TG), HDL-cholesterol (HDL-C) these two groups showed similar values when analyzing the initial values at the first LA session or at an LA session performed years later.
Here we reevaluate the already published data with respect to the focus on reaching the internationally recommended lipid target levels.
Of course, all our patients were on a maximal lipid-lowering therapy in addition to LA – provided the drugs were tolerated.
Table 3 depicts the numbers of patients in the groups who were treated with the listed drugs.
Table 3: Numbers of patients on lipid-lowering drugs in the two groups CVEx/0 and CVEx/1+[13].
Group |
At the start of LA |
In 2019 |
|
Statins |
CVEx/0 |
54 (90 %) |
46 (76.6 %) |
CVEx/1+ |
42 (87.5 %) |
38 (79.2 %) |
|
Ezetimibe |
CVEx/0 |
28 (46.7 %) |
24 (50 %) |
CVEx/1+ |
23 (47.9 %) |
24 50 %) |
|
iPCSK9 |
CVEx/0 |
0 (0 %) |
9 (15 %) |
CVEx/1+ |
2 (4.2 %) |
13 (27.1 %) |
|
iPCSK9: PCSK9 inhibitors (Evolocumab, Alirocumab) |
|||
The statin which was applied most often was Atorvastatin (40 – 80 mg per day). The number of patients who were injecting PCSK9 inhibitors (iPCSK9) was increasing between the two timepoints – start of LA therapy and actual LA session in 2019 – in both groups. The major reason for the initiation of this injection therapy were pre-session LDL-C levels above 1.8 mmol/L (70 mg/dL).
We measured the following LDL-C concentrations in the two groups (Table 4).
Table 4: LDL-C levels (mmol/L; median, IQR) before the first LA session and before and after an LA session in 2019.
Group |
Before 1st LA session |
Before LA session in 2019 |
After LA session in 2019 |
CVEx/0 |
2.07 (1.81 – 3.39) |
2.39 (1.84 – 2.93) |
0.63 (0.47 – 0.87) |
CVEx/1+ |
2.23 (1.68 – 3.36) |
1.91 (1.46 – 2.50) |
0.52 (0.33 – 0.74) |
LDL-C levels were not statistically different between the two CVE-groups at any timepoint.
The data in Table 4 clearly show that LDL-C target levels were reached after the LA session. But it has to be taken into account, that in the days following an LA session LDL-C is constantly increasing. To characterize the LDL-C burden it was recommended to calculate the interval mean values (IMV). For LDL-C, we used the formula published by Kroon [14].
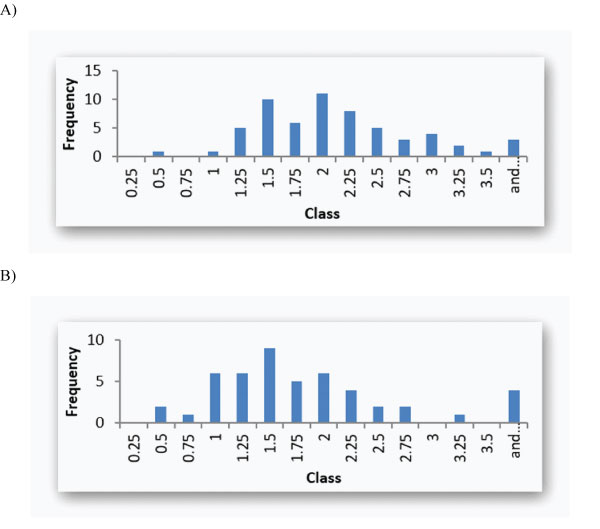
Figure 1: A: LDL-C IMV histogram (mmol/L; upper bounds; median 1.91 (IQR 1.48 – 2.40)) in Group CVEx/0; B: LDL-C IMV histogram (mmol/L; upper bounds; median 1.52 (IQR 1.18 – 2.02) ) in Group CVEx/1+.
Figure 1A and 1B shows the IMV in the two groups.
The histograms depicted in Figure 1 clearly show that only a few patients reached a desirable LDL-C concentration below 1.00 mmol/L in both groups (CVEx/0. 3.3 %; CVEx/1+: 18.8 %). The majority of patients had LDL-C IMV above 1.5 mmol/L. The LDL-C IMV were not different between the two CVE-groups.
One major reason for this problem may be that when measuring LDL-C concentrations in plasma a certain part of them is transported with Lp(a) particles. Some authors suggested to calculate the so-called “true” LDL-C by subtracting the Lp(a)- LDL-C from the measured data. The international trend does not go in this direction for three reasons: 1. We are measuring Lp(a) in nmol/L – you have to convert this into a mass (in mg/dL). There is no generally accepted factor for this; 2. Usually it is assumed that the LDL-C content in Lp(a) is equal to 30 %. It was shown that this percentage may vary; 3. When calculating the “true” LDL-C you get negative concentrations in some patients – most probably due to the uncertainties mentioned.
Table 5: Lp(a) levels (nmol/L; median, IQR) before the first LA session and before and after an LA session in 2019.
Group |
Before 1st LA session |
Before LA session in 2019 |
After LA session in 2019 |
CVEx/0 |
230.5 (159 – 295.6) |
171 (135.5 – 209.3) |
41 (29 – 54.8) |
CVEx/1+ |
235.2 (158.6 – 300.4) |
160 (122 – 211.5) |
38 (29 – 52) |
Now let’s turn to the Lp(a) concentrations observed in the two CVE-groups. (Table 5).
Lp(a) levels at the reported time points were rather similar between the two CVE-groups. Pre-session Lp(a) levels after years of LA treatment were lower than those seen at the first LA session. LA therapy effectively acutely reduced Lp(a) concentrations.
In order to describe the Lp(a) burden we also calculated IMV using a formula which was developed in our group [15].
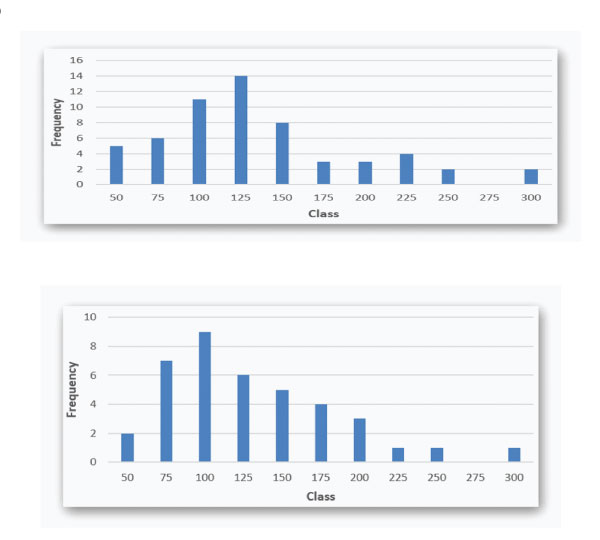
Figure 2: A: Lp(a) IMV histogram (nmol/L; upper bounds; median 115.5 (IQR 90.5 – 149)) in Group CVEx/0; B: Lp(a) IMV histogram (nmol/L; upper bounds; median 102 (IQR 78.5 – 147.5) ) in Group CVEx/1+.
In Figure 2A and 2B, Lp(a) IMV are given as histograms for the two CVE-groups (LA session in 2019).
It appears that Lp(a) IMV are rather similar in both CVE-groups. An Lp(a) target level of 120 nmol/l is exceeded in many patients of both groups. Only a very few reach a “normal” Lp(a) IMV below 75 nmol/l (approximately equal to 30 mg/dl).
In a previous paper we have shown that the achievement of target LDL-C levels less than 1.8 mmol/L (70 mg/dL) in FH patients was 22.6 %, and only 5.7 % patients achieved the target level less than 1.4 mmol/L (55 mg/dL) on statin therapy [10].
Our experience in patients with FH shows, that the target levels for LDL–C on combination with iPCSK9 therapy were achieved in 62.5% of FH patients. Our results are in accordance with other studies evaluating LDL-C target achievement in FH patients [16]. It was shown that the LDL-C target was achieved only in 5.9% of patients [17]. As a result, a significant percentage of patients (55.9%) would be eligible for iPCKS9 treatment. However, even after the addition of iPCKS9 42.4% of patients may still fail to reach LDL-C targets, so, they would thus qualify for a 4th hypolipidemic drug, like bempedoic acid and angiopoietinlike protein 3 (ANGPTL3) inhibitors [17]. In our data 37.5% of FH patients will need a 4th hypolipidemic drug or lipoprotein apheresis, respectively. 40% of our patients needed threecomponent therapy.
Similar results were reported from the PLANET registry, for patients with HeFH in the Czech Republic and Slovakia [18]. In the PLANET registry the target LDL-C level was achieved in 267 patients (15.4%). Among patients who were prescribed iPCSK9, 61.8% had reached the target LDL-C level. In our data the target LDL-C levels on iPCSK9 therapy were achieved in 62.5% of FH patients. Statins can reduce LDL-C up to 50% in HeFH and up to 25% in homozygous FH (HoFH) patients [19] as in our research.
A slight decrease of LDL-C after two times increases in doses of statins is connected with an increased expression of the enzyme PCSK9 [20]. In the Spanish registry SAFEHEARTH (Spanish Familial Hypercholesterolemia Cohort Study) maximal lipid-lowering therapy received 71.8% of patients, and the target LDL-C level <2.6 mmol/l (100 mg/dL) was achieved by only 11.2% of patients [21].
In our data in 37.5% of FH patients the target LDL-C level, corresponding to the risk category, was not achieved. So that patients need a fourth component of hypolipidemic therapy or lipoprotein apheresis. In our data, the main reasons for insufficient effectiveness to achieve target levels of LDL-C when using three- component therapy including iPCSK9 are: in 7% a reduced hypolipidemic efficacy of iPCSK9, in 30% it was doctors mistakes in administration combination of hypolipidemic therapy, in 33% - reasons associated with patients: inability / unwillingness to take high doses of statins, low adherence to lipid-lowering therapy, fear / unwillingness to use multicomponent lipid-lowering therapy; in 30% initially high indicators of the lipid spectrum, which coincides with the results of other researchers [17]. First, the remarkably high baseline LDL-C levels, inherent in FH, make it very difficult or even impossible to decrease LDL-C to new targets with current pharmacotherapy. Second, not all FH patients are treated with high intensity statin and ezetimibe. Third, most patients in the HELLAS-FH were registered before the publication of 2019 ESC/ EAS guidelines. The proportion of patients eligible for iPCSK9 [LDL-C ≥ 1.8 mmol/L (70 mg/dL)] was 51%.
Antibody iPCSK9 have the expected 50–60% reduction in LDL-C, it has become apparent that sporadically a limited LDL-C lowering response is reported. Theoretical mechanisms underlying PCSK9 hyporesponsiveness (<15% reduction in LDL-C) can be divided into two categories: 1) impaired monoclonal antibody entry into the systemic circulation and 2) physiological impairment once the monoclonal antibody is absorbed. Reduced entry of a PCSK9 into the circulation may be related to any of the following: 1) poor adherence to iPCSK9 therapy; 2) improper PCSK9 administration; 3) dermatological factors impairing systemic absorption of drug; and 4) inappropriate antibody disposition [22,23]. With regard to physiological impairment of circulating therapeutic monoclonal antibodies, this may be related to the following issues: 1) mutations that alter the antibody binding site on circulating PCSK9; 2) anti-drug antibodies directed against PCSK9; 3) exaggerated PCSK9 secretion; 4) mutations and/or dysfunctional LDLR, apoB, and/or apoE [22,23]. Вut it is interesting that by far the most common cause of apparent iPCSK9 resistance is related to discontinuation of concurrent lipid lowering therapies (e.g., statins) after initiation of iPCSK9 [23].
The lipoprotein changes in plasma associated with iPCSK9 manifest principally as marked reductions in LDL-C (≈ 50–60%) but there are also notable reductions in Lp(a) (≈25–30%) and TGs (≈ 10–20%) [24,25].The mechanism by which iPCSK9 lower Lp(a) remains unknown. Current hypotheses include [26,27]: increased clearance of Lp(a) particles via the LDLR [28] or via additional receptors (LDL receptor-related protein 1[LRP1] and other receptors [29]; the reduction in apo(a) production, secretion, and/or assembly [30,31,32].
In our opinion, in the future there will be several possible ways to achieve target levels in FH patients and also very high cardiovascular risk patients: 1) the use of multicomponent combined lipid-lowering therapy; 2) increased adherence of patients to lipid-lowering therapy, early detection of nonadherent patients; 3) the use of quadro hypolipidemic therapy in 35-37% of FH patients; 4) the use of pharmacogenetics to detect statin intolerance and insufficient efficacy or use special algorithms for patients receiving iPCSK9 (like suggested by B. Warden [18] for reducing its insufficiency); 5) the use of such components of therapy as LA and new drugs aimed at reducing the level of Lp(a), for example Pelacarsen.
Patients who have started LA therapy are mostly at an extremely high atherogenic risk. They suffered from CVE, most often from several events despite being treated with effective lipid-lowering drugs. Of course, all other risk factors (e.g. hypertension, diabetes mellitus, chronic renal disease, smoking, unhealthy food) have to be optimized.
A prerequisite to start an LA treatment is to teach the patients with respect to eating habits and to apply all available lipid-lowering drugs. Most patients start with statins, and then ezetimibe is added. When problems with statin intolerance appear, bempedoic acid is another therapeutic option (which was started in Germany only 1 year ago). iPCSK9 (monoclonal antibodies or Inclisiran) are indicated in patients who did not reach their LDL-C target values. They may be administered in patients who showed signs of statin intolerability. The extracorporeal LA is the last step in this stepwise approach. But it cannot be overlooked that in some patients CVE may develop though they are treated extracorporeally. We have described that an older age and a higher number of CVE may have a significance for these new events [13].
But in our hands, no differences in lipid concentrations were observed between patients with or without CVE during LA therapy. Nevertheless, we tried to optimize the situation by adding iPCSK9 to the extracorporeal therapy. Of course, this is an expensive procedure.
Though we are combining effective drugs with LA we do not reach the LDL-C target levels in many patients. One reason for this may be that some LDL-C is transported with Lp(a) particles and in this way is not influenced by the commonly available drugs. Yet it was observed (also by us) [26] that iPCSK9 can reduce Lp(a) concentrations.
On the other hand, we do not see CVE in the patients who do not reach the LDL-C targets. This fact casts doubts on the necessity of getting to target in each patient. Evidently, an individualized approach is needed.
For Lp(a) no officially accepted targets have been recommended. Our data clearly point out that LA can effectively reduce elevated Lp(a) levels but will not guarantee a full normalization of Lp(a) concentrations. Thus we are looking forward to the new drugs which will inhibit the synthesis of apolipoprotein(a), like Pelacarsen.
Modern lipidology offers some very effective lipid-lowering drugs which are used in high-risk patients, especially within the framework of familial hypercholesterolemia and of secondary prevention of CVE. Studies with all available drugs and with LA showed that an effective reduction of LDL-C concentrations can be obtained. But in spite of using all these possibilities, even in combination, a major part of our patients will not reach the internationally recommended LDL-C targets. Evidently, this aim may even be not necessary in all patients. For Lp(a) the situation with respect to target levels is at present quite unclear at all.