Breast cancer is the most commonly diagnosed cancer in females and the leading cause of cancer deaths worldwide. Breast carcinomas are categorised into multiple subgroups using well established biomarkers, Estrogen receptor (ER), Progesterone receptor (PgR) and Human Epidermal Growth Factor Receptor 2 (HER2) [1,2]. Different subgroups have distinct phenotype, molecular profiles, clinical behavior and responses to therapy [3]. Hormone receptor positive carcinomas (ER+PR+HER2-) have better prognosis and are treated with endocrine therapy(ER modulator /ER antagonists). HER 2 positive (ER-PR-HER2+) tumours are more aggressive, have low survival rate, do not respond to endocrine therapy and are treated with HER2 blocking agents and chemotherapy. The triple negative subgroup have poor prognosis with short disease free survival and overall survival and are treated with Chemotherapy and targeted therapy (PD-L1 inhibitors). Triple positive subgroup (ER+PR+HER2+), until recently, remained an overlooked subgroup without tailored therapeutic options. These tumours are reported to occur in younger age group, present with larger tumor size and higher stage. Limited studies have shown a higher incidence of lymphovascular invasion and lymphnode metastasis in these tumours [4-7].
Our aim was to evaluate the clinicopathological features of triple positive breast carcinoma in comparison to other subgroups.
Case records of 600 patients who underwent surgical excisions for breast carcinoma which were received in our Pathology department over a 3 year period from May 2017 to May 2020 were collected from the hospital database. 169 cases who had neoadjuvant chemotherapy and in which adequate details were not obtained were excluded from the study. The remaining 431 cases were retrospectively analysed. Patient`s demographic details, presence of comorbidities, history of exogenous hormone treatment and clinical presentations were recorded from the hospital information system. Tumour characteristics such as location, size, WHO histological subtype and Nottingham grade, coexisting ductal carcinoma insitu, presence/ absence of lymphovascular invasion, lymphnode status, distant metastases, TNM stage and hormone receptor status were reviewed from the histopathology reports. Cases were grouped according to receptor status into HR positive, HER2 enriched, triple negative and triple positive and clinicopathological features of triple Positive breast carcinoma were compared with other subgroups.
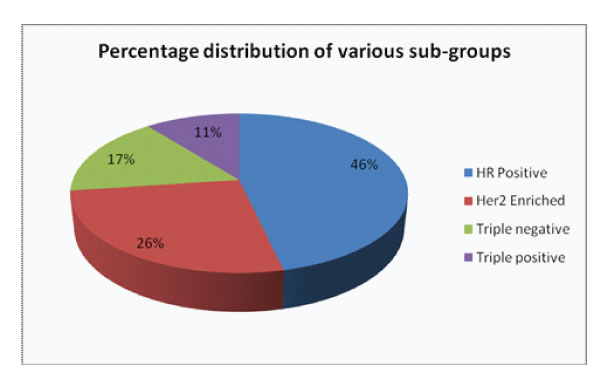
Figure 1: Percentage distribution of various sub-groups
There were 325 modified radical mastectomy and 106 wide local excision specimens. Triple positive subgroup constituted 11% in our study group (Fig.1). These tumours were seen to occur in a relatively younger age group (age range 38-50 years) with 65% cases occuring in patients below 50 years. They were found predominantly on the left side. Patients presented with painless lump, nipple discharge and nipple retraction. Many of them had comorbidities such as Diabetes mellitus, Systemic hypertension, fatty liver, obesity and 9% had exogenous hormonal therapy.
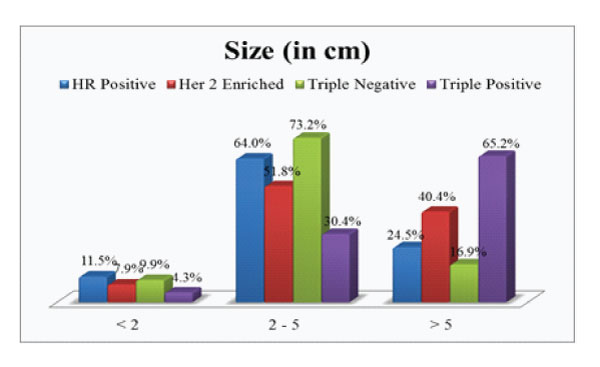
Figure 2: Size of the tumour in each subgroup.
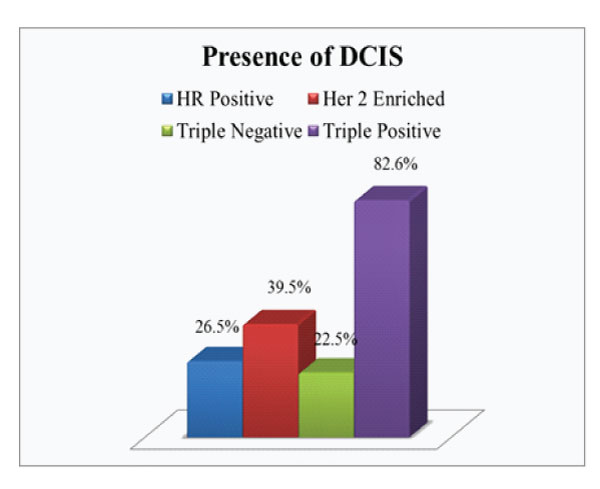
Figure 3: Presence of DCIS in each subgroup
On comparison of the size of tumor in different subgroups, it was found that 65.2% of triple positive tumours presented with large tumor size i.e.>5cm (pT3) which was higher compared to average size in other subgroups (Fig.2). Invasive breast carcinoma of no special type was the commonest histological subtype (97%) with lobular and micropapillary types constituting the remaining cases. 60.9% of these tumours were assigned grade 3. A higher incidence of intermediate to high grade ductal carcinoma in situ (DCIS) were noted in these tumours, seen in 82.6% cases (Fig 3). Lymphovascular invasion and lymph node metastasis were present in 84.8% and 73.9% of our cases respectively, which showed statistically significant difference compared to the other groups (P value < 0.01)(Fig.4&5). Distant metastasis was found only in 13% at the time of initial presentation (Fig.6).
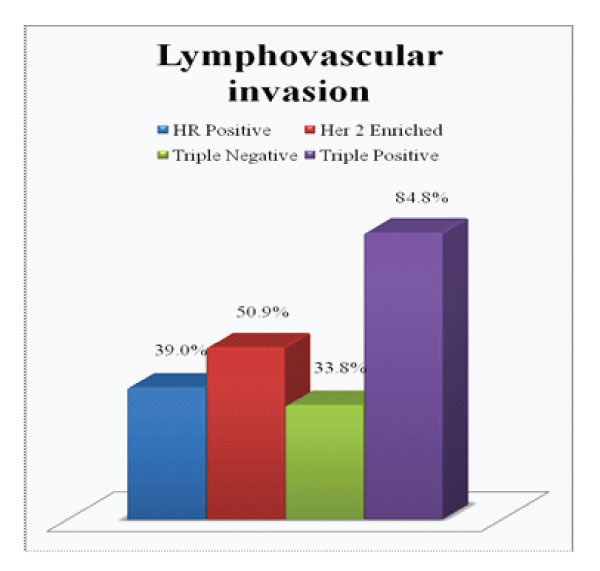
Figure 4: Presence of Lymphovascular invasion in each subgroup
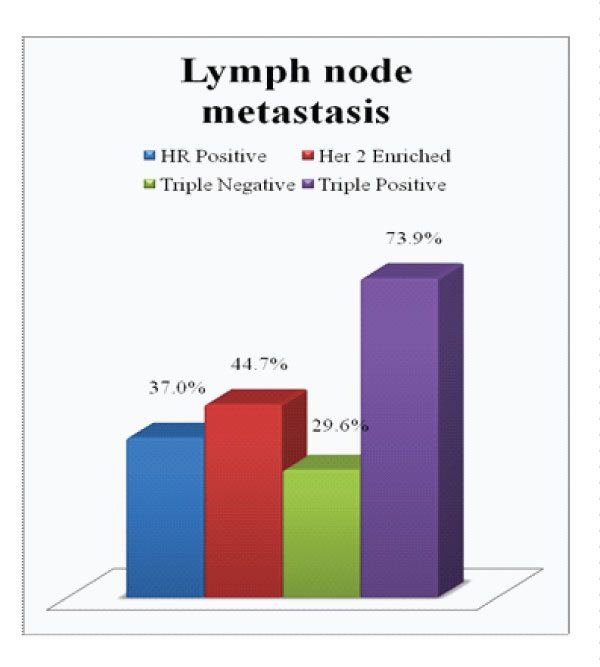
Figure 5: Presence of Lymph node metastasis in each subgroup
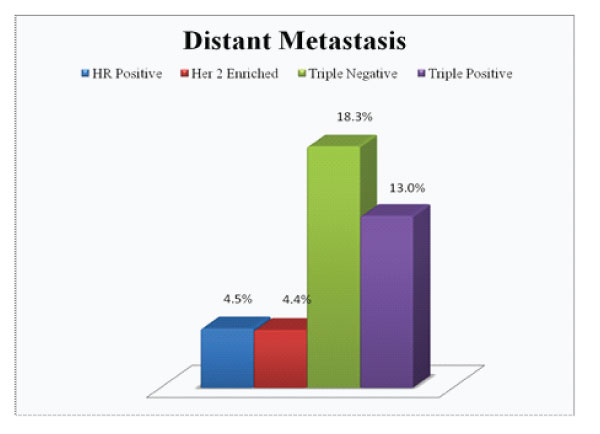
Figure 6: Distant Metastases in each subgroup
Breast cancer is a heterogeneous disease. In this era of targeted medicine, well established biomarkers have played a significant role in improving the therapeutic effectiveness of breast cancer treatment care. ER, PR, HER2 and Ki67 are used as routine biomarkers to diagnose and guide the treatment options. Inspite of all these advancements, HR+ HER2+ tumours have drawn limited attention, compared to other subtypes. Limited studies in the literature have shown that five-year overall survival and disease free survival of HR+ HER2+ patients are worse than that of HR+ HER2− but better than that of HR− HER2+ or HR− HER2−tumours [8,9]. HR+ HER2+ breast cancers have higher chances of being diagnosed in younger populations and as highergrade diseases, compared to other subtypes [10]. These findings support the hypothesis that, not only the clinicopathological, but also biological characteristics of HR+ HER2+ tumours, are different from other subtypes of breast tumours.
Our results concured with the findings in previous studies. These tumours showed a tendency to occur in a relatively younger age group with 65% of our cases occuring in patients below 50 years. Majority of these tumours were >5cm in size which was larger compared to average size in other subgroups. Histological subtype and Nottingham grade were similar to other groups, invasive carcinoma, NST, grade 3 being the commonest. Interestingly, a higher association of intermediate to high grade DCIS were found in triple positive tumours. Also noted were higher incidence of lymphovascular invasion and lymphnode metastasis in these tumours which were found to be statistically significant. However, distant metastasis was found only in 13% at the time of initial presentation, which is less compared to that reported in the literature.
Breast carcinomas being hormone dependent, possibility of an association with fertility medication was considered in view of the higher incidence of these tumours in younger age. However, only 9% in our triple positive group had history of infertility treatment. Studies to date, also have failed to demonstrate a clear causal relationship [11].
All these points shed light to the fact that these tumours are a distinct subgroup and they appear to act differently than other breast cancers in their clinical behavior. Therefore, these patients should be followed up closely for the development of metastasis. Studies also highlight a potential drug resistance due to signalling crosstalk between ER and HER 2 pathways, suggesting a need to tailor therapeutic options specific to this subtype [7].
Eventhough at first glance it seems that triple positive breast carcinomas have better outcome, our study confirms that these tumors present with larger size and higher stage at the time of diagnosis with higher incidence of lymphovascular invasion and lymphnode metastasis compared to other subgroups. Further clinical trials are needed to assess unique behaviour of triple positive tumors to strategise treatment options and potential druggable targets.