Purpose: We evaluated the efficacy and safety outcomes of endoscopic intravesical botulinum toxin A (Botox) injections for the treatment of patients with overactive bladder (OAB) in a single centre with a retrospective analysis.
Introduction: The aims and objectives were to see whether our local practice is in line with international standard in treating patients with OAB symptoms, to evaluate for diagnostic purpose, whether patients have had prior urodynamic test or not, to confirm detrusor overactivity (DOA), to assess the outcomes of treatment in terms of symptoms relief, duration of effect and any repeat injections needed or not and to assess the complications of treatment in comparison with available standards. We retrospectively collected data on all the 9/10 who had undergone this therapy for OAB over a three-year period in a single centre from 2015 to 2017.
Methods: All patients were retrospectively audited from case note review. Data collected consisted included patients Depart ment of Obstetrics and Gynecology, Princess Royal Hospital, University Hospitals Sussex NHS Foundation Trust, Lewes Road, Haywards Heath, RH16 4EX UK admitted for intravesical Botox injections for OAB for three-year period between 2015 and 2017. Selection criteria: A. Patients diagnosed with OAB, not responding to conservative and medical management with at least 2 types of anticholinergic medications. B. All patients had first time treatment in our local hospital. Exclusion criteria- cases who has already had treatment in other units. There were total of 10 patients out of which 9 eligible patients satisfied the audit criteria.
Results: In patient with OAB, significant symptoms improvement was observed n frequency (29%), urgency (38%) and urge incontinent (60%) respectively. Duration of effect vary widely between 3-12 months. All Patients need to be counselled about potential requirement for intermittent self-catheterization (ISC) after the procedure and to attend the ISC session prior to treatment. Procedure can be done under local or general anesthesia. Patients receiving repeated doses do not seem to become refractory to the Botulinum toxin. Complications- mostly injection site pain, procedure-related UTI (Median 3, 32%), mild hematuria (Median 2, 21%), postvoid residual resulting in urinary retention (Median 3, 33%) and requirement for CIC (Median 6-88%).
Conclusions: Treatment with Botox intradetrusor injection seems beneficial and well tolerated with minimal injection site and systemic side-effects in adults with over active bladder resistant to antimuscarinics.
The key aim of therapies targeting the poorly compliant or overactive bladder, whatever the aetiology, is to protect the upper urinary tract from damage [1]. Overactive bladder syndrome (OAB) is a common condition (12-17% of general population) with a significant negative impact on quality of life characterized by urgency with or without urge incontinence, frequency and nocturia. OAB is classified into 2 types; neurogenic, where symptoms are secondary to underlying neurological condition (multiple sclerosis, spinal cord lesions, Parkinson’s disease) and idiopathic, where there is no discernable pathology underlying the symptoms. The standard treatment for overactive bladder starts with patient education and behavior therapies, followed by antimuscarinic agents [2]. Intravesical Botulinum toxin is being increasingly used to treat severe overactive bladder refractory to standard management.
For the period 2015–2017, patients who underwent intravesical Botox procedure in our hospital were analysed. Data are given as percentages or medians (interquartile range). It is a retrospective audit and all case notes were reviewed. Selection criteria: A. Patients diagnosed with OAB, not responding to conservative and medical management with at least 2 types of anticholinergic medications. B. All patients had first time treatment in our local hospital. Exclusion criteria- cases who has already had treatment in other units. There were total of 10 patients and 9 patients were audited as one case was not eligible for audit criteria.
Data collected consisted of age distribution, BMI, annual incidence, associated co-morbidities, smoking status, presenting symptoms, past interventions, urodynamic study results and prior MDT discussion, prior use of medications and types of medications used, type of anesthesia with dose and number of Botox injections, immediate and late complications, symptoms relief after procedure, number of patients with repeat Botox injections, and interval between repeat injections were all documented.
Data were collated and analysed with Excel® (Microsoft, US) and are given as percentages, medians (interquartile range, IQR) or mean (standard deviation, SD).
Most patients were in the age range 50-60 years of age and majority of them had BMI between 30-40. Most cases were seen in 2016. Significant patients had respiratory and associatfied fecal incontinence co-morbidity. All except one patient had smoking habit.
Urgency and urge incontinence were seen in all patients followed by frequency and nocturia. In patient with OAB, significant symptoms improvement in frequency (29%), urgency (38%) and urge incontinent (60%) respectively were noted.
Five patients had prior POP surgery while 3 had USI surgery prior to Botox injections. Urodynamics studies has shown detrusor instability in most patients. All patients had different medications in combination. All cases were done under general anesthesia and had dose of 100 iu of Botox. Duration of effect vary widely between 3-12 months.
Patients receiving repeated doses do not seem to become refractory to Botulinum toxin. Complications- mostly injection site pain, procedure-related UTI (Median 3, 32%), mild hematuria (Median 2, 21%), postvoid residual resulting in urinary retention (Median 3, 33%) and requirement for CIC (Median 6, 88%).
Botox use should be reserved for patients who fail to improve with conservative treatment and medical management with two different anticholinergic drugs. OAB affects 12% to 17% of the population and a third of them experience urinary incontinence, which may greatly impact health related quality of life. Currently both American Urological Association (AUA) and European Association of Urologists (EAU) guidelines suggested that intravesical injection of botulinum toxin A (BoNT/A) should be offered to patients with urgency urinary incontinence (UUI) refractory to antimuscarinic and beta3-adrenoceptor agonist therapy, and the Food and Drug Administration (FDA) approved dose was 100 U of BoNT/A for idiopathic detrusor overactivity (IDO) and 200 U for neurogenic detrusor overactivity (NDO) [3,4]. Optimally safe and effective dose - lower dose 100units have comparable efficacy and improved safety over higher dose (200u/ 300unit).
Number of injection sites should be standardized. Urodynamic test prior to first botox injection should be performed. To consider higher dose of Botox injection if there is recurrence or absence of symptom relief [NICE guidelines, 6]. Practice of training intermittent self-catheterization (ISC) before procedure needs be continued. All cases should be discussed in multidisciplinary meeting (MDM) before treatment. All cases should be reviewed face to face or via phone within 12 weeks of treatment.
Botulinum toxin appears to be an effective therapy to refractory OAB symptoms, but as yet little controlled trial data exists. Further robust data are required on long term outcome, safety and optimal dose of botulinum toxin for OAB [5]. The mechanism of Botox A includes combined effects of the blockade of neurotransmitter release and suburothelial sensory receptors expression.
Suburothelial injection has comparable efficacy for intradetrusor injection. All Patients need to be counselled about potential requirement for intermittent self-catheterization (ISC) after the procedure and to attend the ISC session prior to treatment. Procedure can be done under local or general anesthesia.
Most of the side effects of Botox injections include lower urinary tract complications. Gynecological urology has seen a growing use of Botox in the treatment of refractory OAB and detrusor overactivity (DO). OnabotulinumtoxinA 100 U showed significant, clinically relevant improvement in all overactive bladder symptoms and health related quality of life in patients inadequately treated with anticholinergics and was well tolerated [6,7,8].
Botulinum toxin A significantly improve all symptoms and urodynamic parameters in NDO and OAB in this study, this effect was reported in previous study which demonstrated similar in children and adults alike [1,5]. The effect of Botox A in treating lower urinary tract dysfunction appears to be overestimated in lower as opposed to higher level evidence studies [9]. Botox injections into the detrusor provide a clinically significant improvement in adults with NDO and incontinence/NOAB refractory to antimuscarinics. It seems to be very well tolerated [10].
Therefore, this treatment exhibits very promising risk-to benefit ratio for chronic treatment of NDO/NOAB. However, adequately powered, well-designed, randomised, controlled trials are still lacking and number of questions need to be investigated further. The optimal dose with the longest duration of efficacy and acceptable level of AEs, the timing and indication for repeat injection, the type of patients benefitting most need to be further clarified.
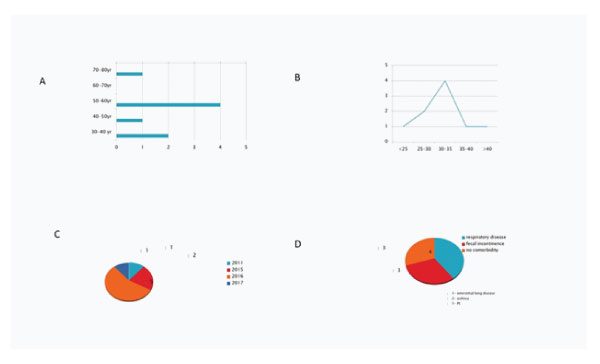
Figure 1: 1A: Age distribution of patients. 1B: BMI distribution of the patients. 1C: Number of cases year wise. 1D: Underlying medical co-morbidity.
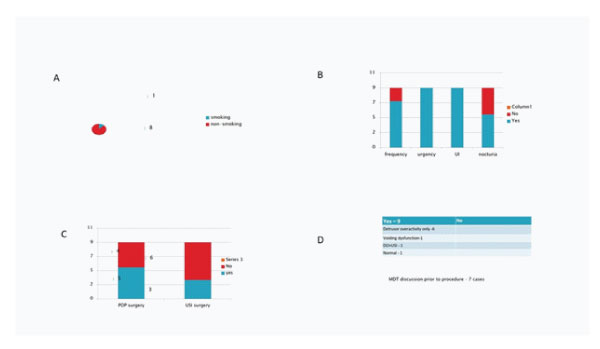
Figure 2: 2A: Smoking status. 2B: Presenting symptoms of patients. 2C: Number of cases year wise. 2D: Underlying medical co-morbidity.
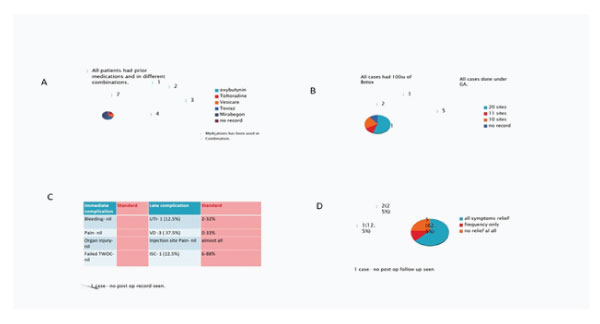
Figure 3: 3A: Prior use of medications and types of medications used. 3B: Anesthesia, dose and number of Botox injections. 3C: Immediate and late complications. 3D: Symptom relief after procedure (quantitative).
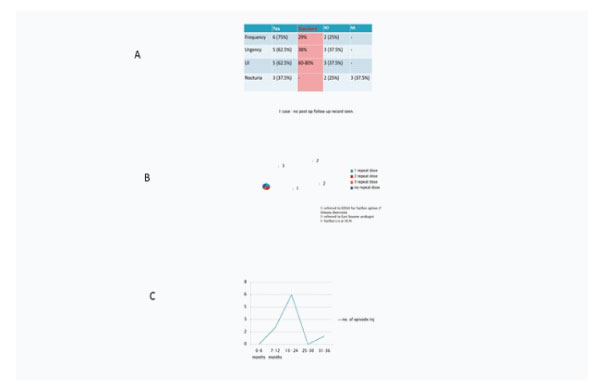
Figure 4: 4A: Symptom relief after procedure (qaulitative). 4B: Number of patients with repeat Botox injections (No. 8). 4C:Interval between repeat injections.
Treatment with Botox intradetrusor injection seems beneficial and well tolerated with minimal injection site and systemic side-effects in adults with over active bladder resistant to antimuscarinics.
Conflict of interest
The authors have no conflict of interest to declare. No funding source was involved in this study.
Ethical approval
All procedures performed on human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the parents of each child prior to all the procedures. All parents were informed about the procedure.