Aim:Adjuvant therapy was introduced in 2018 for patients with sentinel node biopsy positive melanoma. The aim of this study is to compare the outcome of completion lymphadenectomy versus adjuvant therapy for patients with positive sentinel lymph node biopsy (SLNB) in cutaneous melanoma.
Materials and Methods: All patients with primary cutaneous melanoma who underwent wide local excision of melanoma scar and SLNB between January 2014, and December 2020 were included in the study. Patients with positive SLNB before October 2018 were offered lymphadenectomy and patients with positive sentinel lymph node after October 2018 were reviewed by oncology and offered adjuvant therapy if suitable.
Results: 202 patients underwent SLNB during the study period. 14.35% (29 patients) had a positive sentinel node. The average Breslow thickness was 2.1mm in the negative SLN group and the average Breslow thickness was 2.74mm in the positive SLN group.
Patients with sentinel node positive melanoma treated by completion lymph node clearance had a recurrence rate of 33.33% which is higher than the rate of recurrence in patient who received adjuvant therapy (25%).
Keywords: Melanoma, Sentinel Node Biopsy, Adjuvant Therapy.
Abbreviation:
SLNB: Sentinel lymph node biopsy
NICE: National Institute for Health and Care Excellence
The European annual incidence of malignant melanoma varies from 3–5/100 000 in Mediterranean countries to 12–35/100 000 in Nordic countries, whereas it can reach over 50/100 000 in Australia or New Zealand. The incidence of melanoma has been rising steadily over the last forty years, with a trend towards stabilisation of mortality, except in elderly males [1].
Since the first introduction of Sentinel Lymph node technique in 1992 by Drs Donald Morton and Alistair Cochran, minimally invasive intraoperative lymphatic mapping and sentinel node biopsy has become the standard approach for staging the regional lymph nodes for early-stage melanoma [2,3].
Sentinel lymph node status has been shown recently to be by far the strongest independent prognostic factor in melanoma stage I-II patients i.e. those that are node negative and SLN-status is a much stronger prognostic factor than tumour thickness, which loses its prognostic relevance in SLN-positive patients [6]. In patients with a melanoma of Breslow thickness >4mm, approximately 30% of patients will have a positive sentinel node [4,5].
The most recent updated NICE guidelines from 2015 for the use of SLNB it state that clinicians should consider sentinel lymph node biopsy as a staging rather than a therapeutic procedure for people with stage IB–IIC melanoma with a Breslow thickness of more than 1 mm, and give them detailed verbal and written information about the possible advantages and disadvantages of the technique. They should not offer imaging or sentinel lymph node biopsy to people who have stage IA melanoma or those who have stage IB melanoma with a Breslow thickness of 1 mm or less [7].
AJCC melanoma staging version 8.0 enables the classification of patients into different categories of risk for disease recurrence. Increasing Breslow thickness, presence of ulceration and increased mitotic rare are all associated with an increased risk of relapse [12]. Prior to its introduction in 2018 our unit offered sentinel node biopsy to all patients with a Melanoma >1mm Breslow thickness but after these updated staging guidelines were published sentinel node was offered to all patients with a Breslow thickness >0.8mm [8,9]. Patients with pT3b melanoma (2-4mm Breslow thickness with ulceration) and above undergo CT staging prior to sentinel node biopsy to exclude distant metastases before surgery in this higher risk group. Patients are not offered sentinel lymph node biopsy if they are not fit for general anaesthetic and have a careful discussion regarding the role of sentinel biopsy if they have co-morbidities which will exclude them from adjuvant therapy. If not suitable for adjuvant therapy they may still have sentinel biopsy for the prognostic information it provides and to make them eligible for imaging surveillance as part of their follow up protocol.
Five adjuvant therapy trials have now reported: EORTC 18,071, CheckMate 238, EORTC1325/Keynote 054, Combi- AD and Brim-8. The inclusion criteria, based around AJCC version 7 rather than version 8.0, varied between the trials, ranging from Stage IIC to Stage IV resection. All five trials showed a significant improvement in relapse-free survival; however, three of the trials are too immature to report on overall survival [10].
A variety of factors influence the decision to offer adjuvant therapy and guide the choice of systemic anticancer therapy. Factors include the risk of disease recurrence, stage at diagnosis, BRAF mutation status, and patient characteristics including age, comorbidities, and personal preferences. If appropriate, the patients should also be encouraged to participate in clinical trials.
Patients with low-risk node-positive disease (stage IIIA) are usually reviewed by the oncology team and a careful discussion held explaining the risk and benefits of adjuvant treatment versus surveillance. Data indicates that these patients are at a lower risk of disease recurrence and have five year relapse free survival (RFS) of over 90% [13]. For patients with stage IIIA disease and BRAF-mutated primary cancer, who are keen for treatment, adjuvant targeted therapy with one year of dabrafenib plus trametinib is a reasonable option to surveillance. BRAF targeted therapy has manageable side effects and the COMBI-AD trial [14] demonstrated improved overall survival (OS).
High-risk node-positive disease (stage IIIB, IIIC, IIID) remain at significant risk for disease recurrence even after surgical resection [15]. Adjuvant systemic therapy should be discussed with these patients. Treatment should be offered in order to reduce the risk of recurrence and improve overall survival. For patients with resected, high-risk node-positive disease lacking a BRAF V600 mutation, one year of adjuvant immunotherapy with a programmed cell death-1 inhibitor (PD-1) is discussed. Systemic anticancer agents include either nivolumab [16] or pembrolizumab [17] as both improved RFS and were well tolerated.
Other systemic therapies such as ipilimumab and interferon have been investigated for use in the adjuvant setting, however due to the lack of efficacy and risk of severe toxicity are not used in the U.K.
The use of immunotherapy in patients with stage IIB and IIC is being assessed and results may change future practice.
The purpose of our study is to evaluate all the patients with stage III melanoma and positive SLNB treated in our hospital from 2014 to December 2020.
Study characteristics
A retrospective observational study was carried out. We included all patients with melanoma who underwent SLNB at Poole General Hospital, from January 2014 to December 2020.
The following was recorded from the Electronic Patient Record.
The patient data was analysed in 2021 which meant length of follow up varied between 1 and 6 years.
In our study, recurrent disease is defined as distant metastasis, the presence of melanoma in a lymph node basin, or local recurrence at the primary site.
Lymphatic Mapping and Surgical Technique:
The day before, or the morning of surgery, all patients underwent preoperative lymphatic mapping with 0.5-1 mCi of Tc99 m injected intra-dermally at the site of primary melanoma. The SLN was identified by the nuclear physician using a handheld gamma probe and then marked on the skin with a permanent marker. Immediately preoperatively 2 ml of Patent Blue dye was injected intra-dermally at the site of primary melanoma scar. All basins identified as radioactive were explored through limited incisions directed by the handheld gamma probe. The SLN was defined as the node with the highest radioactive count. This node was identified and removed, and it was sent in formaldehyde solution directly to the Department of Pathology. Any node(s) with >10% count rate of the most radioactive node was also removed and analysed. Intraoperative SLN histological analysis was not performed on any patient. Patients also underwent a simultaneous wide local excision of the primary melanoma site with margins excised according to current guidelines.
Histologic Analysis of Surgical Specimens:
The submitted SLN were reviewed by routine histopathological study (Haematoxylin and Eosin) and by immune-histochemical methods (S-100 and Human Melanoma Black 45). A macrometastasis was defined as >2mm of melanoma within the sentinel node. A micro metastasis was defined as <2mm of melanoma within the node.
Overall results
A total of 202 Sentinel Node Biopsies were performed and of these 29 were positive for melanoma (14.35%). This data is shown in Figure 1. The average Breslow thickness was 2.1mm in the negative SLN group with the majority having a pT2a tumour as illustrated in Figure 2. The average Breslow thickness was 2.74mm in the positive SLN group with the majority having pT3 tumours as shown in Figure 3.
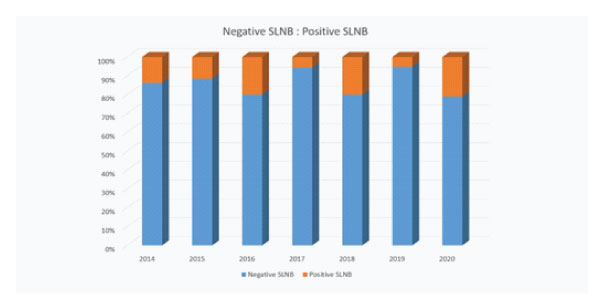
Figure 1: Graph to show percentage of positive and sentinel nodes.
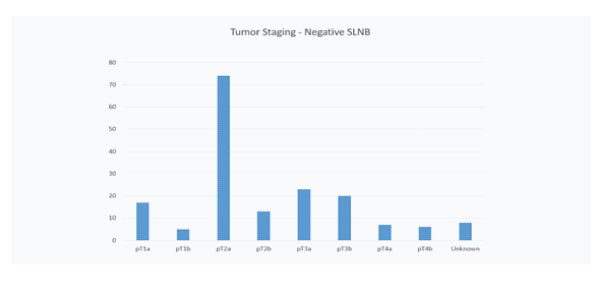
Figure 2: Graph to show tumour staging of primary melanoma in patients with a negative sentinel node.
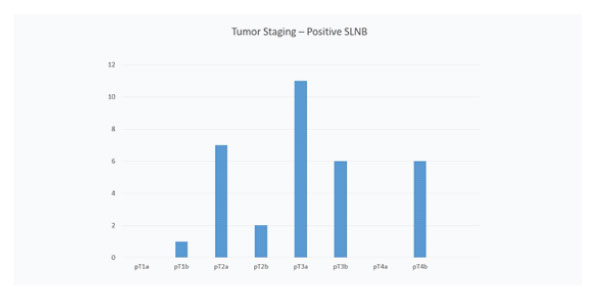
Figure 3: Graph to show tumour staging of primary melanoma in patients with a positive sentinel node.
Analysis of Patients before Introduction of Adjuvant Therapy:
Before the introduction of adjuvant treatment in late 2018 the total number of patients who underwent sentinel node biopsy was 125. Of these 15 had macro-metastasis within the sentinel node (12%) with an average Breslow thickness of 2.50 mm, and went on to have completion nodal clearance, 2 patients had micro-metastasis which didn’t require further treatment.
Follow up data from the sentinel node positive patients (n=15) who had nodal clearance, showed 4 died from melanoma recurrence, 1 had recurrence but is still alive, 2 were lost follow up and 8 patients are alive with no evidence of recurrence. In this group, the recurrence rate was 33.3% with an average Breslow thickness of 2.50 mm.
Analysis of Patients after Introduction of Adjuvant Therapy:
Since the introduction of adjuvant treatment in late 2018, 77 patients have undergone sentinel node biopsy out of which 11 proved to have macro-metastasis with the sentinel node (14.3%), with an average Breslow thickness of 2.84 mm, 3 of these were not eligible for adjuvant treatment due to their medical conditions. One patient had micro-metastasis in the node and did not require further treatment.
Follow up data from the group of patients (n=11) who had macrometastasis in the sentinel node confirmed that 2 died from melanoma recurrence, 1 had recurrence (mot eligible for adjuvant) and 6 alive with no evidence of recurrence. The recurrence rate in this group of patients was 25%
Table 1: Overall outcomes for patients with a positive sentinel node
Positive SLNB |
Treated |
Recurrence Rate |
Died from Melanoma |
Recurrence |
Alive with No recurrence |
|
Patients treated prior to adjuvant therapy |
17 |
15 |
33.33% |
4 |
1 |
8 |
Patients treated after introduction of adjuvant therapy |
12 |
8 |
25% |
2 |
0 |
6 |
We accept the limitations of this study include the small number of patients and that some patients have been lost to follow up. This is not a formal randomised controlled study but an observational study due to change in management as new guidelines have been introduced. The aim of the study was to see if there was improved survival in the group of positive sentinel node patients treated with adjuvant therapy compared to those treated with completion lymphadenectomy. The overall results have shown improved outcome in patients treated with adjuvant therapy compared to those who had nodal clearance, unfortunately the numbers are too small to perform meaningful statistical analysis. The improvement in survival is in addition to sparing the patients complications from nodal clearance surgery such as lymphoedema, seroma, nerve damage and long term pain which can be quite debilitating for some patients.
The length of follow up varies between patients with the longest being 6 years and the shortest 1 year so long term follow up data is needed to see if the difference in recurrence rate persists at 10 years.
Our results indicate that the current guidelines recommending adjuvant therapy in preference to nodal clearance in patient with positive sentinel nodes should be followed as they offer patients the best prognosis. In this study, patients with stage III melanoma treated by completion lymph node clearance had a recurrence rate of 33.33% which is considerably higher than the rate of recurrence in patient who received adjuvant therapy (25%). In conclusion, in this observational study adjuvant therapy showed significant longer recurrence-free survival than nodal clearance and avoided further surgery and associated surgical complications.
NICE Melanoma: assessment and management. NICE Guide 2015 nice.org.uk/guidance/ng14.
Sondak VK. ASCO Educational Book. In. 2017.
Nivolumab for adjuvant treatment of completely resected melanoma with lymph node involvement or metastatic disease. NICE Technology appraisal guidance 2021 nice.org.uk/guidance/ta684.
Weber J, Larkin J, Mandala M, Gogas H, Fernandez A, Dalle, S, et al. Five-year outcomes with adjuvant nivolumab versus ipilimumab in resected stage IIIB–C or IV melanoma (CheckMate 238). Presented at the Society for Melanoma Research October 28-21, 2021.