Purpose: We aimed to present our experience within similar to laminoplasty with a simple method technique that allows stabilization of the spinal cord without instrumentation during tumor surgery.
Methods: This retrospective study was performed in the neurosurgery department and data were collected from the medical files of 26 patients (12 females, 14 males) who were treated surgically for spinal tumors. The same surgeon operated the patients using the same procedure without any instrumentation for stabilization of the spinal cord. Demographic and clinical data were collected. It was checked whether radiological and clinical instability developed at the 6th and 12th months postoperatively.
Result: Our series comprised 26 patients (12 females, 14 males) with an average age of 51.12 ± 20.33 (range: 19 to 86 years). The most common complaints were bilateral paresis of lower extremities (20, 47.62%), radicular pain (9, 21.43%), and hypoesthesia (2, 4.76%). The most frequent histopathological diagnoses were schwannoma (8, 19.04%), ependymoma (7, 16.67%), meningioma (6, 14.28%), and metastatic carcinoma (5, 11.90%), respectively. The most involved levels were L1-L2 (5,11.90%), L2 (4, 9.52%), and T5-T6-T7 (2, 4.76%).
Conclusion: In spinal tumor surgeries, we think that with the suture technique, which takes a very short time without instrumentation, the spine stabilization can be preserved and it will shorten the operation time and provide great comfort for the patient. However, longer periods of follow-up, as well as prospective, controlled, multi-centric trials on larger populations, are warranted to evaluate the safety and efficacy of novel technique.
Keywords: instrumentation, spinal tumor, surgery, stabilization, suture, laminoplasty
Surgery has significant benefits over other treatments to resolve spinal cord compression, alleviating pain, and improving quality of life while posing few risks in the management of primary and metastatic tumors of the spinal cord [1,2].
Decompression and stability are the main goals of spinal surgery. Typically, the procedure serves only as a palliative measure. Surgery is occasionally utilized for curative care of primary and, even more rarely, secondary tumors. Surgery is preferred (1) when there are neurologic complications related to local tumor development with local compression or fracture, (2) when there is a mechanical complication with a fracture or axial destabilization, (3) when pain does not respond to medical treatment, surgical treatment of spinal metastasis is recommended, (4) in cases of radioresistant cancers (e.g., renal cell carcinoma that does not respond to chemotherapy or radiotherapy; tumor recurrence after prior radiotherapy). We must carefully consider suitable biomechanical qualities for the three columns during reconstruction [3].
In the treatment of spinal malignancies, ensuring spinal stability and mobility is a difficulty that typically necessitates meticulous surgical planning [4]. The treatment of spinal metastatic illness includes spinal stabilization surgery. Because of their overall prognosis and concurrent therapy, patients with spinal oncology are unlikely to achieve bone fusion. For these patients, stabilization surgery without fusion may be a viable option. There is a scarcity of research on the effectiveness of this strategy [5]. One of the parameters in the evaluation of surgery outcomes is stabilization. This is a valuable and important parameter. While there are many effective parameters such as morphometric parameters and CSF dynamic parameters [6,7].
Although spinal tumors are treated using a variety of surgical procedures, the goal of each treatment, however, is the same: to restore spinal stability and decompress neural tissues, such as the spinal cord and nerve roots [8]. The traditional surgical approach in the treatment of spinal tumors was laminectomy, which can provide an adequate surgical field. However, this surgical approach is associated with some complications such as spinal deformity and spinal instability [9]. Laminoplasty, which reconstructs the lamina using instruments such as a titanium plate, T-saw or translaminar screw, is widely applied in patients with spinal tumors [12]. Some studies have shown that collapse and displacement of the laminae may occur in patients undergoing laminoplasty. In osteoporotic or elderly patients, the fixed titanium screw may loosen easily and cause secondary spinal stenosis, resulting in spinal cord injury. [11]. We aimed to share our experience with our novel operative technique that allows stabilization without instrumentation in the surgical management of spinal tumors together with a brief review of current literature.
Study design
This retrospective study was carried out in the neurosurgery department of a tertiary care center after the approval of the local institutional review board (2021/26/03). Data were gathered from the hospital database of 26 patients (12 females, 14 males) with an average age of 48.12 ± 20.33 (range: 19 to 86 years) treated surgically for spinal tumors. The baseline descriptives, clinical and radiological information, operative and histopathological data were recorded. A single senior neurosurgeon (IA) carried out the consecutive spinal procedures in our center during a 3-year period (between 2017 and 2021).
Patients with spinal tumors who underwent laminectomy were included in the study. Hemilaminectomy or had significant preoperative instability were excluded from the study. Patients with multiple myeloma were excluded from this study because this disease has biological characteristics that differ from metastatic lesions and solid tumors, especially in terms of the probability of bone repair [8].
Surgical procedure
In our novel surgical method, we maintained stability without instrumentation during surgery for spine tumors. Total laminectomy was performed on the vertebral laminae required according to the location and level of the tumor. intradural or extradural tumor was removed.supraspinous and intraspinous ligaments were sutured with pds sutures (figure 1a,b,c,d,e).The patients were given a corset or cervical collar for 2 months in the postoperative period. Since the medial aspects of facets and tension bands were preserved, no instability was detected during follow-up on the 6th and 12th months after surgery. It was checked whether radiological and clinical instability developed at the 6th and 12th months postoperatively.
Outcome parameters:
The variables under investigation included age, sex, radiological data derived from magnetic resonance imaging (MRI), neurological findings, the level of surgical procedure, histopathological diagnoses and radiological instability.

Figure 1a,b,c: Sagittal illustration of surgery.
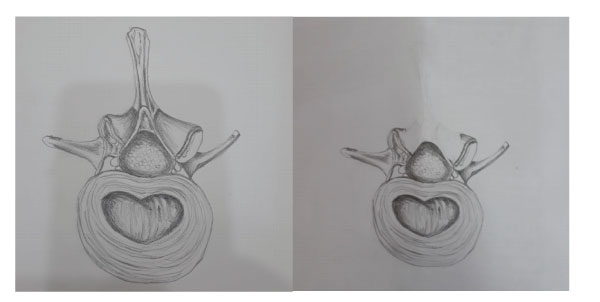
Figure 2a and 2b: Axial illustration of surgery.
Statistical analysis
The data were analyzed using Statistical Package for Social Sciences program version 20.0 for Windows (SPSS, Inc., Chicago, Illinois, USA) program. Descriptive data were expressed as mean, standard deviation or median, minimum, and maximum values for quantitative variables, while categorical variables were shown as numbers and percentages.
Our series comprised 26 patients (12 females, 14 males) with an average age of 51.12 ± 20.33 (range: 19 to 86 years). The most common complaints were bilateral paresis of lower extremities (20, 47.62%), radicular pain (9, 21.43%), and hypoesthesia (2, 4.76%). The most frequent histopathological diagnoses were schwannoma (8, 19.04%), ependymoma (7, 16.67%), meningioma (6, 14.28%), and metastatic carcinoma (5, 11.90%), respectively. The most involved levels were L1-L2 (5, 11.90%), L2 (4, 9.52%), and T5-T6-T7 (2, 4.76%). No instability was detected during follow-up on the 6th and 12th months after surgery. Magnetic resonance imaging was performed routinely to evaluate the extension of spinal tumors. An overview of detailed demographic, clinical, radiological, and histopathological data is demonstrated in Table 1.
This study describes the patient group in which spinal stability is preserved without instrumentation in patients who were operated for a spinal tumor. Analysis of data collected from our patients yielded promising results with this novel procedure. The significance and worth of this procedure will become obvious as we gather further knowledge and competence about the indications and efficacy of our unique way of surgery for tumors of the spine through larger research. This method allows avoidance of instrumentation failure and associated morbidity due to additional interventions for fusion and instrumentation
Table-1: Overview of descriptive, radiologic, clinical and histopathological data in our series (n=26).
No. |
Sex |
Age |
Magneticre sonance findings |
Neurological examination |
Level of pathology |
Histopathological diagnosis |
1 |
M |
46 |
A 25X12 mm lesion without contras tenhancement in the spinal canal at the level of T4 |
Bilaterallowerextremities, motor power 1/5 |
T4 |
Fibrousmeningioma |
2 |
M |
42 |
Intradural, extramedullary lesion with contrast enhancement in the spinalcanal at thelevels of T4-T5 |
Bilateral motor power at lower extremities: 2/5 |
T4-T5 |
Meningioma |
3 |
M |
42 |
Intradural, extramedullary lesion of 1.5X1.5 cm at the levels of T12-L1 |
Radicularpain at right lower extremity |
T12 |
Myxoidschwannoma |
4 |
F |
63 |
Intradural, extramedullary lesion with contrast enhancement at the level of T7 on rightside |
Neurogenicclaudication |
T7 |
Meningioma |
5 |
F |
39 |
Extradurallesionordisc at the levels of T10-T11 |
Bilateral motor power at lower extremities: 3-4/5 |
T10-11 |
Hyalinized, ischemicchondroid tissue fragments |
6 |
F |
41 |
A lesion of 18X13 mm without contrast enhancement at thelevel of L2 vertebra |
Constipationandurinaryretention |
L1-2 |
Mature cysticteratoma |
7 |
F |
63 |
Intradural, intramedullary lesion with contrast enhancement at the levels of T10-T11 |
Right footdorsiflexion: 2/5 |
T10-T11 |
Astrocytoma |
8 |
M |
29 |
Sixextramedullary lesions (thelargestonewith a size of 21X14 mm) with homogeneous contrast enhancement |
Bilateral motor power at lower extremities 2/5, hypoesthesia |
T3-T7 |
Meningioma |
9 |
F |
58 |
Intraaxiallesion of 13X7 mm in the spinal cord of at the level of T2-T3 with moderate and heterogenous contrast enhancement |
Radicularpain, anthalgicgait |
T2-T3 |
Ependymoma |
10 |
F |
43 |
Intradural, extramedullary lesion of 47X20X15 mm in spinal cord with hypointense contrast enhancement at T1A and mild hyperintense contrast enhancement at T2A sections at the level of L4 vertebral body. |
Hypoesthesia at right lower extremity |
L3-L4 |
Myxopapillaryependymoma |
11 |
M |
69 |
A soft tissue mass that extend stopedicle and transverse process on left and toward spedicle on the right leading to fracture and loss of height at the level of L2 vertebra body with contrast enhancement and hypointense T1A, isointense T2A and hyperintense STIR sequences. |
Bilateral motor power at lower extremities : 3/5 |
L2 |
B-celllymphoidneoplasia |
12 |
M |
42 |
Intraaxial lesion in the spinal cord with moderate heterogeneous contrast enhancement at the levels of T3-T4. |
Bilateral motor power at lower extremities: 1/5 |
T3-T4 |
Ependymoma |
13 |
F |
23 |
A lesion of 27x24x19 mm at thelevel of T1 vertebra with contrast enhancement. |
Bilateral motor power at lower extremities: 2/5 |
T2-T3 |
Meningioma |
14 |
F |
19 |
Intramedullary cystic lesion displaying compression on the junction of cauda and spinal cord at thelevel of L1-L2 without contrast enhancement |
Bilateral motor power at lower extremities: 3/5 |
L1-L2 |
Neuroentericcyst |
15 |
M |
45 |
Intradural, extramedullary lesion of 8X5X6 mm at thelevel of L2 at right posterior neighbourhood of vertebra with contrast enhancement |
Radicularpain at left lower extremity |
L2 |
Schwannoma |
16 |
M |
69 |
L4-5 An intradural lesion of 14X10X9 mm within spinal canal with smooth margins at the level of L4-L5 lesion displaying intense contrast enhancement |
Radicularpain |
L4 |
Schwannoma |
17 |
M |
39 |
Intramedullary lesion at levels of C2-3 |
Motor power at bilateral upper and lower limbs: 4/5 |
C2-C3 |
Ependymoma |
18 |
M |
46 |
Intradural, extramedullary bilobulated and communicating lesions with the largest size of 47X13 mm at thelevel of C2-C3 with significant contrast enhancement and isointense appearance at T1 and T2A. |
Neckpain |
C2 |
Transitionalmeningioma |
19 |
M |
79 |
Cysticlesion of 8 mm in the posterior part of spinal cord at thelevel of T7-T8 without contrast enhancement and hyperintensity at intradural T2A views. |
Bilateral radicular pain, bilateral motor power at lower extremities: 3/5 |
T7-T8 |
Hyalinizedfibrocollagenous connectivetissue with focal deposition of calcifiedhemosiderin pigment |
20 |
F |
69 |
A lesion of 40X37X35 mm extending from right posterior part of vertebra body to right pedicle, laminae and transverse process at the level of T9-T10 with intense contrast enhancement. |
Bilateral motor power at lower extremities: 2/5 |
T9 |
Metastaticcarcinoma |
21 |
M |
86 |
A lesion of 23X20X16 mm at thelevel of T2 verterbra body with contrast enhancement. |
Bilateral motor power at lowerextremities: 2/5 |
T2-T3 |
Schwannoma |
22 |
F |
58 |
Lesion at thelevels of T5-T7 extending towards ribs on the right side and posterior lytopara spinal muscles. |
Bilateral motor power at lowerextremities: 1/5 |
T5-T6-T7 |
Malignantepithelialtumorinfiltration |
23 |
M |
15 |
Intradural, intramedullary lesion of 3X1 cm within spinal cord causing remarkable dilatation of thes pinal canal at the levels of C3-C5 without contrast enhancement and isointensity at T1A sequences, mild hyperintensity at STIR A sequences. |
Motor power at bilateral upper and lower extremities: 3/5 |
C4-C5 |
Ependymoma |
24 |
M |
68 |
Lesion of 18X8 mm at thelevel of T5 posterior to the right side of vertebra body with mild contrast enhancement and heterogeneous nodularity. |
Motor power at right lower extremity: 1/5, left lower extremity: 2/5 |
T4-T5 |
Metastaticcarcinoma |
25 |
F |
70 |
Well-circumscribed intradural lesion of 16X12x8 mm in spinal canal at the level of L4-L5 with homogeneous and intense contrast enhancement. |
Radicularpain |
L4 |
Schwannoma |
26 |
F |
68 |
Bilobulated and communicating intradural, extramedullary lesions of 47X13 mm remarkable contrast enhancement and isointensity at T1 and T2A sequences. |
Radicularpain |
T11-T12 |
Schwannoma |
Abbreviations: F: female; M: male; WHO: World Health Organization; C: cervicalvertebrae; T: thoracicvertebrae; L: lumbarvertebrae |
||||||
on spinal bones that are already fragile due to adverse effects of chemotherapy and corticosteroids on bone mineral density. In our series, the average duration of follow-up was 12 months.
Postoperative radiation and chemotherapy are commonly given to patients with spinal metastatic illness, which can hinder new bone development [12]. Corticosteroid therapy can lower bone mineral density and impair osseous healing potential [13]. The dietary deficiency seen in cancer patients may enhance the occurrence of complications associated with instrumentation and fusion more likely [12].
The utility of our technique, which avoids instrumentation, may diminish the operative time and amount of blood loss. Moreover, the risk of destabilization of the spinal column due to posterolateral decortication as a part of fusion will be omitted [14]. The likelihood of stimulation of tumor cells by instruments or fusion substrate may be avoided. The lack of instrumentation will save an important cost and decrease the financial burden [15]. The implementation of surgical outcome studies can be difficult when studying a condition with a low survival rate [16]. Although there is a growing tendency toward minimally invasive and short-segment constructions, such surgical procedures can lead to failure of instrumentation [17].
Patients with spinal metastatic illness are diverse. Because of their unique features, such as pathophysiology, chemotherapy regimens, and metastasis, they constitute a tough population to examine. The goals of treatment vary remarkably in these patients [5].
When there is progressive discomfort owing to spinal instability, or when either vertebral collapse or metastatic development produces spinal cord compression, surgical therapy is recommended [18]. Instability-related pain and loss of motor power are the most common presenting symptom, and it was noted in the vast majority of our patients.
As a result, when considering whether surgery is the best treatment, the realistic aim of pain control must be considered. The second major purpose of surgery is to decompress neural tissues so that neurologic function can be restored or preserved [8]. In patients with spine tumors, the more intensive treatment seems not to have much effect on survival [8].
Thus, less invasive, safer, and more practical procedures may be more suitable in this selected group. The expected survival rate is critical during selection of the surgical intervention in patients with metastases. In comparison to the conservative care, various studies have shown that even palliative surgery can improve the prognosis in such individuals [19]. When compared to the radiation monotherapy group, a higher percentage of operated patients kept or regained their ability to walk and required lower corticosteroid and analgesic doses, shifting the therapeutic paradigm for spinal tumors [20]. Patients with spinal tumors may have various primary pathologies, shorter survival times, and inconsistent follow-up due to morbidities or debilitation, as well as exposure to a variety of pharmacologic drugs. Therefore, surgical techniques, instruments, and radiation protocols differ significantly in this subgroup [20].
Primary spinal cord tumors ((SCT) are relatively uncommon in adulthood, accounting for just 2% to 4% of all primary central nervous system cancers [21,22]. Extradural SCTs account for 60% of all SCTs, 30% of intradural SCTs, and 10% of SCTs with both intradural and extradural components [4]. Within the
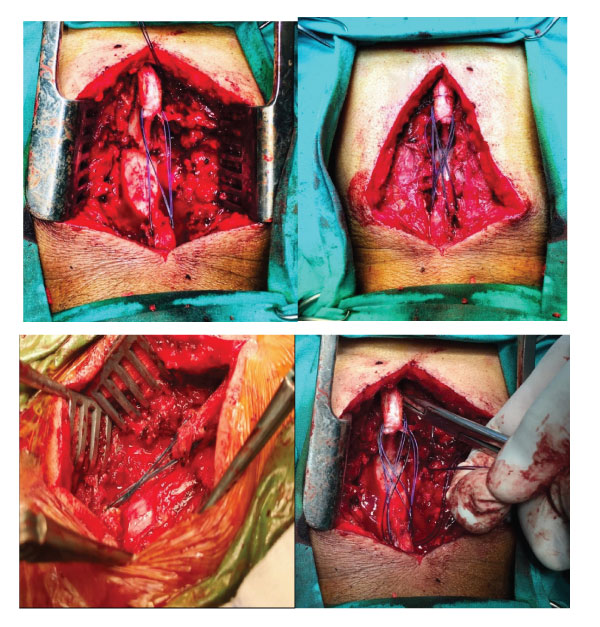
Figure 3a,b,c,d: Intraoperative views demonstrating our technique for stabilization of the spinal cord without instrumentation after excision of tumor.
intradural space, SCT can be divided into two types: intradural extramedullary (70%) and intradural intramedullary (30%) [21,22]. The distribution of our series was similar to these aforementioned reports.
The guidelines for fixation and instrumentation in the management of patients with spinal tumors do not currently exist. Total surgical excision is the chosen treatment for SCT because it has the best long-term results [23]. Some surgical interventions such as facetectomy [24,25] and surgery involving a spinal junction [26], are well documented to cause instability after spine surgery. Minimally invasive surgery for spinal tumors is a valuable procedure that can successfully generate good clinical results while reducing non-surgical costs when appropriate surgical indications exist [21,27]. It's crucial to achieve a balance between the short-term morbidity risks of vigorous resection and the longterm recurrence risks of incomplete resection.
Patients with spinal tumors need a careful surgical technique and patient specific evaluation based on cost-effectivity and minimizing risks associated with additional morbidity due to malignancy. Resection of the lesion and preservation or restoration of structural integrity of the spine must be taken into account during tailoring the treatment plan [4].
Instrumentation, fusion and other interventions may bring about additional risks for the fragile structure of the spine in patients with tumors. Thus, we speculate that our method can be a safe and straightforward surgical alternative in selected cases with spinal tumors.
The most prevalent location of metastatic bone disease is the spinal column. More than 90% of spinal cancers are metastatic lesions, with the most common sources of metastasis being the lung, breast, prostate, and kidney [28,29,30]. The thoracic and thoracolumbar spines are the most common sites for metastases inside the spinal neural axis (70%), followed by the lumbar spine
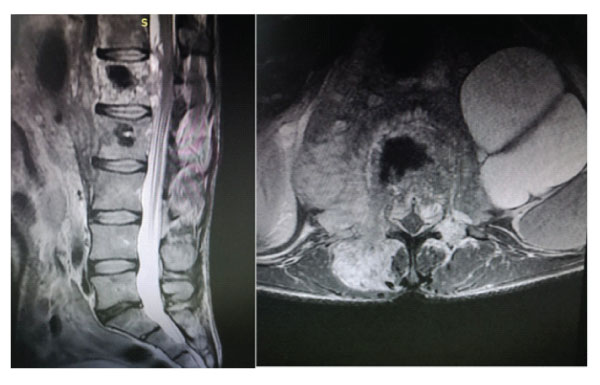
Figure-4: Sagittal and axial MRI images of the patient before surgery.
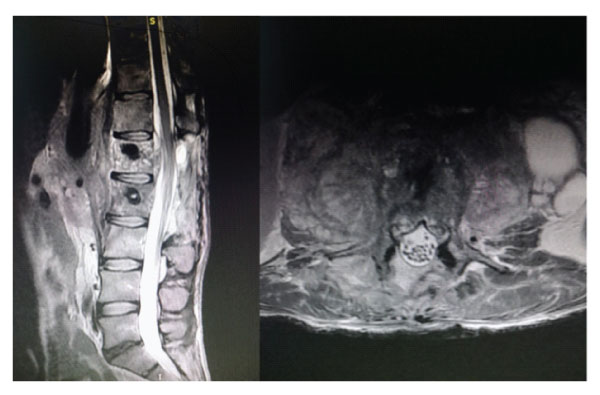
Figure-5: Sagittal and axial images of postoperative early control MRI.
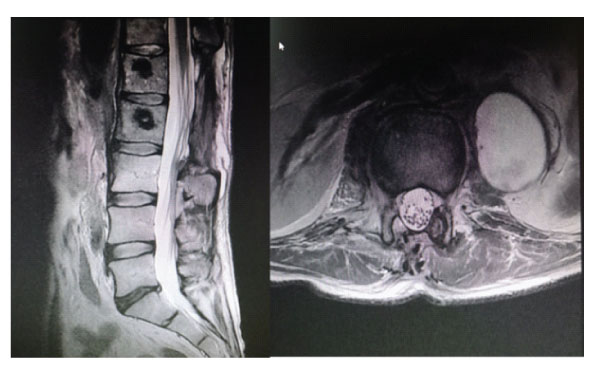
Figure-6: Sagittal and axial images of postoperative 1st year follow-up MRI.
and sacrum (20%), and the cervical spine is the least common [31]. Our results are in parallel with this report.
Neural compression and spinal fracture can occur as a result of spinal metastases, resulting in excruciating pain and neurologic impairment. Decompression surgery and spinal stabilization are crucial in the treatment of spinal metastatic disease [30].
Attributed to their overall prognosis and concurrent medications, the goals of care for spinal oncology patients may differ from those for non-oncology patients [32,33]. Patients with spinal metastatic disease may not live long enough to achieve bone fusion or experience hardware failure [34,35]. As a result of constant chemotherapy, radiation therapy, and low nutritional status, their bone's healing potential is frequently impaired [36].
Pain reduction, neurologic function preservation, prevention of progressive spinal deformity, and improved overall survival and quality of life are all goals of spine stabilization in oncology patients. Fusion may not be necessary to achieve these aims in patients with spinal metastatic disease [28]. While the spine is being fused, instrumentation can only stabilize and maintain its alignment. Therefore, the implanted hardware cannot replace bony parts of the spine [37]. Instrumentation for the establishment of stability may result in loosening or fracture due to the amount of stress at the bone-screw interface, that may lead to instrument loosening or fracture [38]. There is a scarcity of research on the effectiveness of spinal stabilization without fusion or instrumentation in individuals with tumors of the spine [5]. Laminoplasty, which reconstructs the lamina using instruments such as a titanium plate, T-saw or translaminar screw, is widely applied in patients with spinal tumors [10]. Some studies have shown that collapse and displacement of the laminae may occur in patients undergoing laminoplasty. In osteoporotic or elderly patients, the fixed titanium screw may loosen easily and cause secondary spinal stenosis, resulting in spinal cord injury. [11]. in our study; Similar to the purpose of laminoplasty, we have ensured that stability is maintained without the use of instrumentation. Also there were no additional new deficits or pain, as there would be no posterior elements and instrumentation that could compress the chord when lying in the prone position. Spinal instability was not observed in patients who underwent laminectomy at 2 levels or more with the surgical technique we applied. We anticipate that our technique will pave the way for more research into better surgical planning, care safety, patient outcomes, and cost-cutting in the medical treatment of patients with spinal tumors. However, some limitations of the current study must be remembered during the extrapolation of our results to larger populations. Retrospective and single-center design, lack of a control group and evaluation of the quality of life after surgery, relatively short duration of follow-up and possible impacts of socioenvironmental factors and ethnicity are weaknesses of this study.
To conclude, although the treatment of spinal tumors is difficult, satisfactory and effective results can be obtained with effective and short-term surgery suitable for patients. Complications and recurrence must be minimized to the greatest extent feasible, emphasizing the importance of thorough preoperative evaluations, precise surgical techniques, and knowledge based on previous resections or surgical procedures. The suturing technique we have described has shown promising results in patients, as it shortens the operative time and maintains stability without instrumentation. However, further multi-centric trials on larger series are warranted to achieve more accurate conclusions.
Acknowledgments
None
Statement of ethics
We declare that this research complies with the guidelines for human studies and it was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Source of funding
No financial support was received for this paper.
Author contributions
İdiris Altun: design of the study, collection of data, statistical analysis, writing, critical revision, and approval of the final version of the manuscript.