Cervical syringomyelia is usually associated with Chiari 1 malformation, spinal tumor posttraumatic or post-infection conditions. However, CSF flow disturbance at the foramen magnum level without tonsillar or hindbrain herniation can cause the syringomyelia, although limited cases have been reported in the literature. In this report, we present two cases of cervical syringomyelia without tonsillar or hindbrain herniation, in which clinical and radiological resolution of syringomyelia was observed following foramen magnum decompression.
Keywords: Cervical syringomyelia, Chiari 0, foramen magnum decompression.
Syringomyelia is the presence of fluid filled cavity extending longitudinally within the spinal cord [1]. If it is associated with dilated central canal of spinal cord, it is called as hidromyelia. Syringomyelia usually occurs in the cervical region and is associated with Chiari malformations characterized by herniation of cerebellar tonsils below the foramen magnum more than 3-5 ms. Spinal intramedullary tumors and arachnoiditis secondary to trauma and infections are other causes of syringomyelia. Syringomyelia, which is not associated with any pathological condition, is considered as idiopathic syringomyelia. Foramen magnum decompression is the most accepted and well-known treatment of cervical syringomyelia associated with Chiari malformations although syringomyelia secondary to trauma or infection and idiopathic syringomyelia can be managed by shunting the syrinx [1,2].
However, CSF flow disturbance at the foramen magnum level without tonsillar or hindbrain herniation can also cause syringomyelia, although limited cases have been reported in the literature [3,5].
Case-1
A 45 years old woman was admitted to the hospital with a history of progressive weakness and numbness of the upper extremities. She also had a headache worsening with coughing and gait disturbance. Neurological examination revealed spasticity and weakness of extremities with hyperreflexia. Paresthesia, decreased pain and temperature sensation under C3 level were also observed. Lateral X-rays showed bony abnormality at the craniovertebral junction without basilar invagination. Dynamic
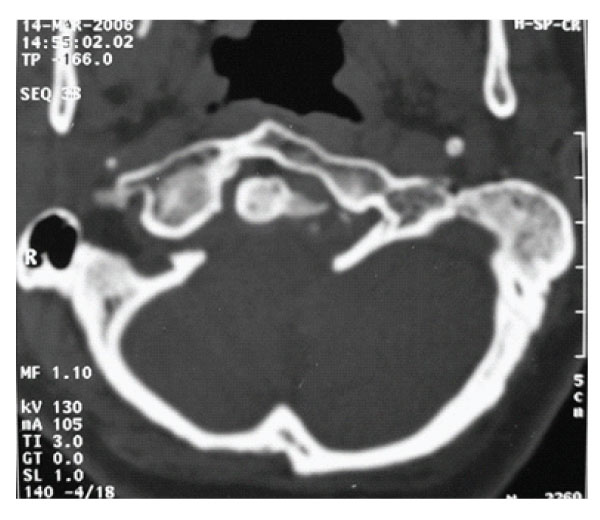
Figure 1: Axial slice of CT scan showing anterior assimilation of Atlas and asymmetric condyles.
X-ray examination disclosed no instability. Axial CT scans, three dimensional reconstructed CT scans and MRI showed anterior occipitalization of atlas, asymmetrical condyles with hypoplasia of left posterior part of the atlas, rightward deviation of odontoid process and C1-5 syringomyelia without tonsillar herniation [Figure- 1,2,3,4]. Posterior part of the axis was also asymmetrical. There was no appearance of basilar invagination although the tip of the axis was 2 mm superior to the foramen magnum level (McRae line).
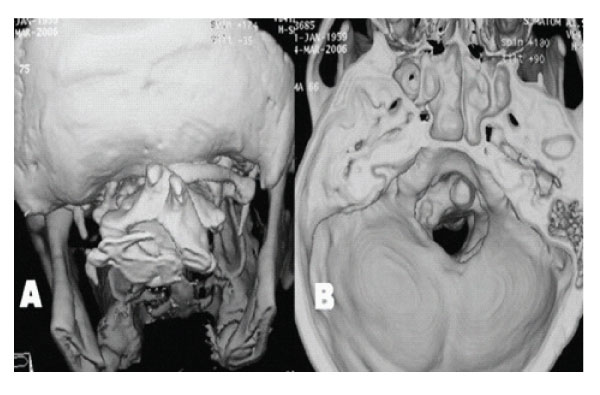
Figure 2: A: 3 D reconstructed CT scan showing assimilation of right posterior arch of Atlas, its right posterior arch can be seen. B: 3 D reconstructed CT scan showing inner aspect of posterior cranial fossa. A narrow, asymmetrical foramen magnum and shallow posterior fossa are seen.
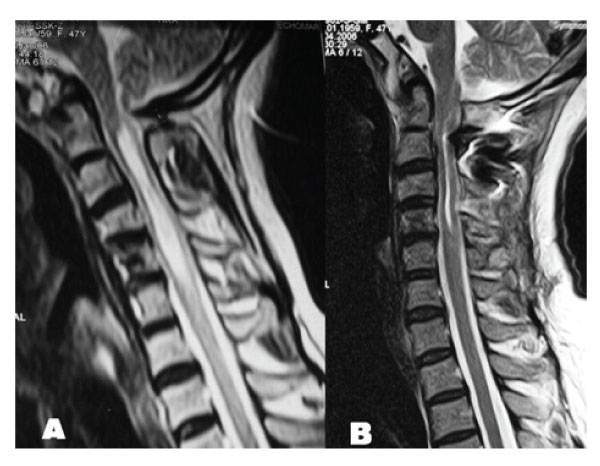
Figure 3: A: T2 weighed sagittal MRI demonstrating cervical syringomyelia without tonsillary herniation. B: Postoperative T2 weighted sagittal MRI demonstrating resolution of cervical syringomyelia.
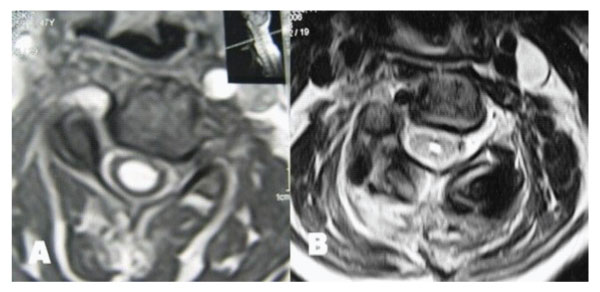
Figure 4: T2 weighted axial MRIs taken at C5 level demonstrating resolution of syringomyelic cavity. A: Preoperative. B: Postoperative.
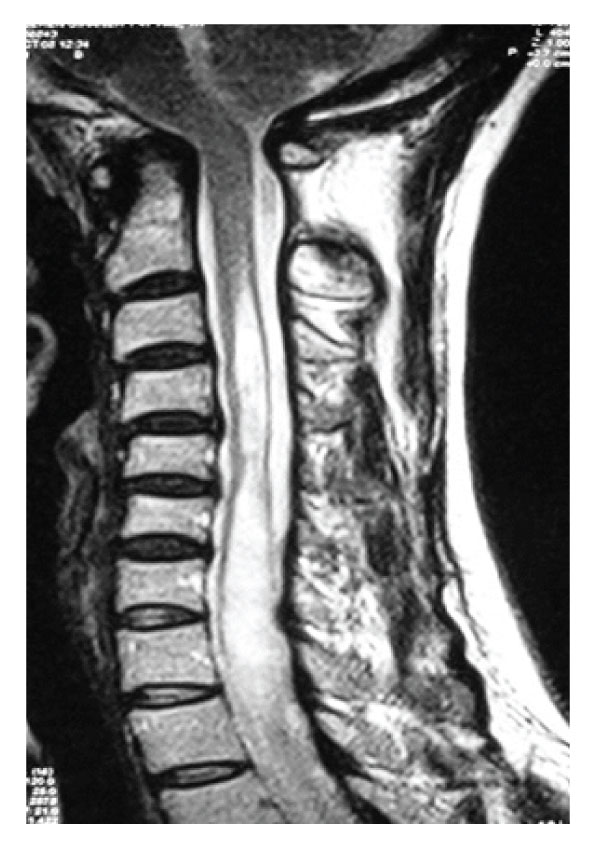
Figure 5: Case 2. Preoperative T2 weighted sagittal MRI of cervical spinal cord revealing syringomyelia below C2 level without tonsillary herniation.
Antero-posterior diameter of foramen magnum was 20 mm; no bony compression to neural structures including odontoid process was detected, however anterior subarachnoid space could not be seen at the level of brain stem. MR angiography disclosed aplasia of left vertebral artery with a nearly normal trace of the right vertebral artery. The patient was treated by a posterior fossa decompression. In the operation, suboccipital craniectomy was performed. The right lamina of the atlas was also removed. Dural surface became expanded and pulsatile suggesting foramen magnum stenosis. CSF flow was observed after opening the dura mater. A large duraplasty was made with dural graft to maintain the CSF flow. Following the decompression and large duraplasty, occipital cervical fusion using C2 laminar screwing was performed. Post-operative period was uneventful, spasticity and weakness of the extremities and hyperreflexia improved. MR examination obtained six months after the operation showed the resolution of the syringomyelia [Figure 3,4].
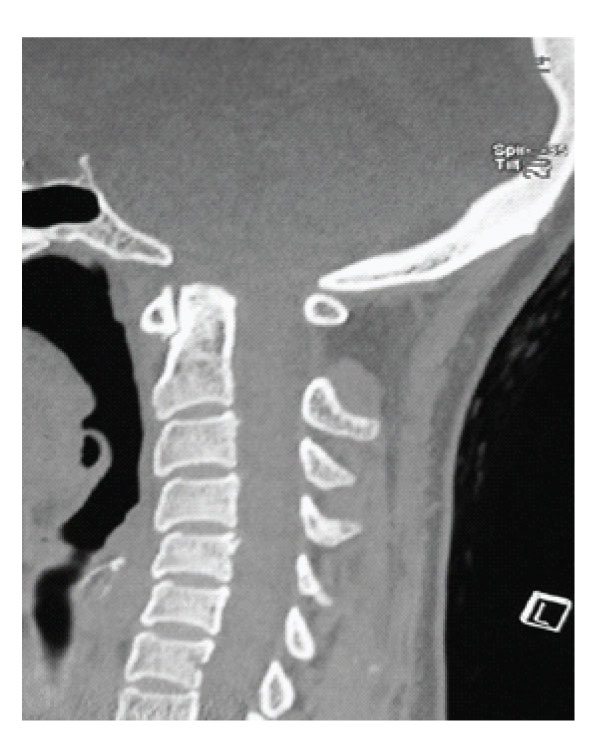
Figure 6: Case 2 Pre-operative Sagittal CT scan showing short clivus and short basis occiput associated with small posterior fossa.
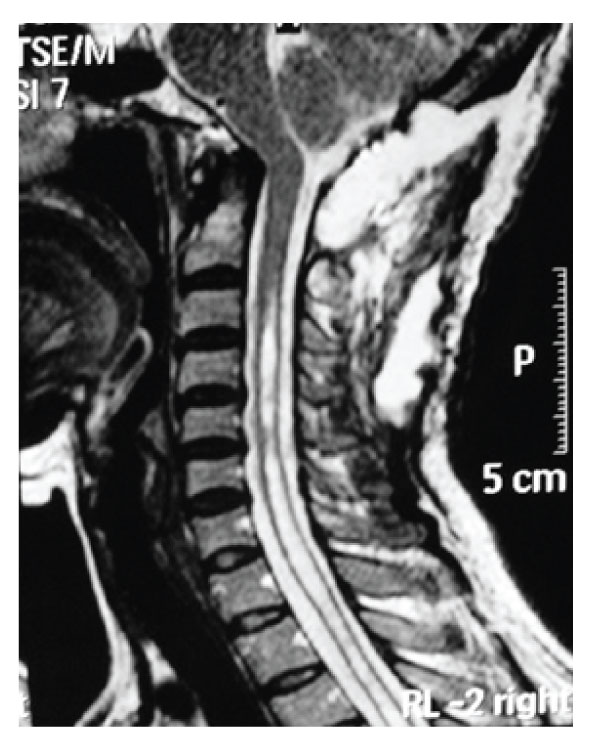
Figure 7: Case 2 Post-operative T2 weighted sagittal MRI demonstrating resolution of the syringomyelia.
Case-2
A 48 years old woman presented with a 5-year history of progressive headache and numbness on her upper extremities. She also suffered from weakness in both hands and gait disturbance. Her symptoms became worse over a 6 months period before admission. Neurological examination revealed slight quadriparesia with tendon hyperreflexia on the upper and lower extremities. Under C6 level, paresthesia and decreased temperature sensation without muscle atrophy were detected. MRI revealed a small posterior fossa with C4-Th6 syringomyelia without tonsillar herniation [Figure-5]. In CT scan of craniovertebral junction, short basis of occiput and short clivus with increased angle of tentorium was detected [Figure-6]. Dynamic plain films of the patient did not demonstrate instability. The patient was treated via posterior fossa decompression with C1 laminectomy and duraplasty. Intraoperative exploration disclosed no intradural adhesions. However, CSF flow was observed following dural opening. Postoperative period was uneventful and symptoms were gradually improved. Three months after the surgery the control MRI revealed decreased syringomyelia under C4 level [Figure-7].
In 1981, Hans Chiari described four types of cerebellar anomalies which are called Chiari malformations [6]. Today Chiari I malformation is described as caudal displacement of cerebellum tonsils to a level below the foramen magnum, while Chiari II malformation involves caudal displacement of cerebellar vermis, brainstem and fourth ventricle. In contrast to the Chiari type I, Chiari type II malformation is usually associated with myelomeningocele, hydrocephalus and intracranial anomalies. One of the most common manifestations of Chiari malformation is syringomyelia. Several pathogenic theories have been proposed to explain the development of syringomyelia associated with Chiari malformation, although no theory of formation of syringomyelia has been universally accepted [1,3]. However, the main cause of the syringomyelia is thought to be an obstruction of the CSF circulation at the foramen magnum [1,2]. Herniated cerebellar tonsils are responsible for the foramen magnum compression in the cases of Chiari I, while caudal herniation of the cerebellar vermis, brainstem and fourth ventricle cause the foramen magnum obstruction in Chiari II cases. Before the CT and MR era Newton described syringomyelia cases without hindbrain herniation, in which posterior fossa decompression were effective [7]. Similar cases have been described as “forme fruste of Chiari malformations”, “syringomyelia without tonsillar ectopia” or “significant descent of tonsils”. In large series of syringomyelia associated with Chiari I malformation, although CSF pathway at the foramen magnum level was open, surgeons found adhesions, which obstruct the fourth ventricular outlet. Batzdrof reported a patient with a partial membraneous fourth ventricle obstruction in association with cervical syrinx, but without cerebellar ectopia [8]. In 2002, Kyoshima et al. reported four cases with syringomyelia without tonsillar herniation [5]. They found that the subarachnoid space anterior to the brain stem usually was found to be open however cisterna magna was impacted by the tonsils and they called this situation as “thight cisterna magna”. In two of these cases, accompanied ventricular dilatation was observed probably due to impaired CSF draining at the level of foramen Luschka. In all these cases, main problem was CSF flow obstruction at foramen Magandie with a veil or compression of cerebellar tonsils.
Klekamp et al. also reported 21 cases of forman magnum arachnoiditis with cervical syringomyelia without tonsillar herniation [9]. 18 of the 21 cases were operated and severe arachnoid scarring was found in these cases. Most of the patient had a history of meningitis, hemorrhage, birth injury or subarachnoid hemorrhage. However, in two cases the main cause was foramen magnum stenosis.
In 1998, Iskandar et al reported five pediatric cases of syringomyelia without tonsillar or hindbrain herniation, successfully treated with posterior fossa decompression [3]. Afterwards the same group reported the measurements of posterior fossa in these cases with an additional one and formed the idea that a compromised or distorted posterior fossa and the caudal displacement of brain stem caused a ‘crowded’ foramen magnum that was responsible for the development of syringomyelia [10,11]. They called these cases as Chiari 0 malformation. In two cases, patients had bone anomaly (posterior defects of C1 and C2, and assimilation of atlas on the right), while the others had intradural pathologies such as adhesion or veil tissue. However, foramen magnum diameters of these cases were not smaller than the average of control group. In our cases, patient 1 had a complex craniovertebral junction anomaly including anterior occipitalization of atlas, deviation of odontoid process and hypoplasia of condyles. Atlantoccipital assimilation can be unilateral, segmental or bilateral, and its incidence is as low as 0.25% [12]. In most of the cases, other craniovertebral junction anomalies such as basilar invagination and Klippel Feil syndrome accompany. However, only 5.2% of symptomatic Chiari I cases have occipital bone anomaly including occipitalization of the atlas, condylar hypoplasia [12]. Recently, Kagawa et al reported a case of Chiari I malformation accompanied by assimilation of the atlas, Klippel Feil syndrome and syringomyelia, and found only one similar case in the literature [12].
In 1999, Milhorat et al. reported clinical and radiological findings of 364 patients with CMI [1]. They found that patients had reduced posterior fossa space and CSF volumes although there was no significant difference in the mean brain volume of posterior fossa, compared with control groups. Other MRI findings were short clivus and supraocciput, kinking of medulla, retroflexion of odontoid and increased angle of tentorium. However, only small number of the patients had tonsillar herniations less than 5 mm. Milhorat et al. also concluded that the extent of tonsillar herniation could not be used as the sole criterion for the diagnosis of CMI.
More recently Sekula et al. evaluated the morphological features of the posterior fossa in 22 nonsyringomyelic patients with Chiari-like symptoms (cephalgia, neck pain, lower cranial nerve deficits etc.) and without tonsillar descent 3 mm below the foramen magnum [13]. Measurements of posterior fossa parameters include lengths of the clivus, basiocciput, basisphenoid and degree of the tentorium angle. This study was disclosed that patients with Chiari-like symptoms had a smaller posterior fossa than that of normal population. These results suggested that a subgroup of patients exists without apparent of tonsillar herniation but with hypoplasic posterior fossa causing Chiari-like symptoms. The term of “Chiari 0” could be appropriate to this situation. In 2015 Goel proposed that Chiari I malformation was secondary to the potential or manifest atlantoaxial instability [14]. He also speculated that tonsillar herniation was a natural protective process in which they act just like an airbag to protect the critical neural structure and reported case series of syringomyelia associated with basilar invagination and Chiari I treated with only atlantoaxial fixation. According to Goel a group of atlantoaxial instability (ones with posteriorly located facets of atlas and ones diagnosed only clinically and intraoperatively) is associated with syrigomyelia and Chiari I. In 2017, Goel’s group also reported 9 cases of syringomyelia without tonsillar herniation treated with atlantoaxial fixation [15]. These cases were similar to our cases but considered as idiopatic syringomyelia although atlantoaxial instability was diagnosed by CT in 8 of them. However, our case 1 supports the theory of Goel but the second does not. The main obstacle against the Goel’s theory is the expectation of clinical detoriation, due to increase of atlantoaxial instability following posterior fossa decompression if it really exists.
Therefore, a group of patients associated with syringomyelia without tonsillar herniation, which can be treated with posterior fossa decompression exists [Table 1].

Table 1: Possible causes of cervical syringomyelia without apparent tonsillar descent.
However, every patient could not be put only in one group. For example, a patient with hypoplasic posterior fossa may also have a foramen magnum obstruction with bony anomaly or a veil at foramen Magendie level. Our second case had a smaller posterior fossa suggesting “Chiari 0” although no intradural veil was found.
Pathogenesis and developmental mechanism of syringomyelia with Chiari I malformation is identical with that of syringomyelia associated with obstruction at the level of foramen magnum without tonsillar herniation. In two groups, main cause of syringomyelia is the CSF obstruction at foramen magnum level and both of them could be defined as foramen magnum lesion related with syringomyelia.
Radiological examinations including plain radiographs, CT or MRI may give information, such as small posterior fossa (short clivus, increased angle of tentorium), craniovertebral bony anomaly, open or closure of anterior CSF pathways or bulging of the upper cervical cord (indirect sign of arachnoiditis). Cine MRI is the most useful radiological examination showing CSF blockage at the level of foramen magnum [16,17]. However, Struck and Haughton reported cine MRI findings of 8 cases of idiopathic syringomyelia and found CSF obstruction at the foramen magnum level in 4 of them but, syrinx resolution following posterior fossa decompression was observed in only one case [16].
Posterior fossa decompression which is pathophysiology based treatment of cervical syringomyelia associated with Chiari I malformation is effective and widely accepted. Syringomyelia not associated with any definite pathogenic lesion is usually named as “idiopathic syringomyelia” managed by shunting of the syrinx. As in our cases syringomyelia with CSF obstruction at the foramen magnum level without tonsillar herniation should be treated by posterior fossa decompression with duraplasty. Because, these cases are not true idiopathic syringomyelia cases and shunting is usually ineffective. Following osseous decompression with intradural exploration should be carried out and CSF flow should be observed before closing the dura.
In the cases with craniovertebral junction bony anomaly, a special attention must be paid to the bony structure, the abnormal course of vertebral arteries and the mechanic stability of the craniovertebral junction. In case 1 we also performed occipitocervical stabilization to prevent the development of instability. Surgical outcome is usually satisfactory in the cases of Chiari 0 although in the cases with tight posterior fossa, extensive syringomyelia and foramen magnum arachnoiditis considerably have worse outcomes
Only a small group of patients with cervical syringomyelia, associated with CSF obstruction at the level of foramen magnum without tonsillar herniation exists. This group is presented with the same clinical symptoms as that of Chari I malformation and the physiopathology of this situation is identical with that of syringomyelia associated with Chiari I malformation. Possible causes are forme fruste of Chiari malformation (Chiari 0), tight posterior fossa and foramen magnum arachnoiditis. Cine MRI is essential to demonstrate CSF obstruction at foramen magnum level. Recognizing this group of patients is crucial because craniocervical decompression to maintain the CSF flow is the treatment of choice and shunting procedures are usually ineffective.