Aims: A sedentary life, smoking, obesity, and psychological stress can modulate the immune response to viral infections. However, the association between these risk factors and the pathogenesis of viral myocarditis remains unanswered. Therefore, the study aimed to evaluate if these factors increase the susceptibility to viral myocarditis in young adults.
Methods: The study population consisted of 40 young adults diagnosed with acute virus myocarditis confirmed by cardiac MRI (Group A). In addition, 40 controls with symptoms similar to myocarditis but negative MRI confirmation were selected (Group B). All patients and controls filled out a self-report questionnaire, which evaluated their body weight estimation and behavioral characteristics, such as physical activity, smoking habits, and anxiety.
Results: We demonstrate a significant association between sedentary life, smoking, and high-stress levels with the risk of viral infection leading to myocarditis.
Conclusion: Common risk factors in the lifestyle of young people were suggested to impact the appearance of acute virus myocarditis in young people.
Keywords: Viral Myocarditis, Viral Infection, Young People, Lifestyle
Acute viral myocarditis is an inflammatory disease of the heart resulting from viral infections and post-viral immunemediated responses [1]. Myocarditis is responsible for about 5%-20% of cases of sudden death and nonischemic dilated cardiomyopathy among young adults [2]. Αpproximately 1% to 5% of patients with an acute viral infection, usually respiratory or gastrointestinal origin, may exhibit myocarditis [3]. The spectrum most frequently detects viruses shifts from classic enteroviruses, such as Coxsackie B virus and adenoviruses, to mainly parvovirus B19 and human herpesvirus 6 [4,5]. Also, acute myocarditis was often reported as a proportion of cardiac injury in patients with COVID-19 [6]. The pathogenesis of viral myocarditis includes the direct damage mediated by a viral infection and the indirect lesion resulting from host immune responses [7]. Genetic factors such as the existence of specific receptors in myocardial cells. e.g., coxsackievirus-adenovirus receptor might be involved in the exhibition of myocarditis [8]. The innate and myocardium-specific adaptive responses generate an autoimmune process responsible for developing myocarditis and progression to dilated cardiac myopathy [9].
Engaging in moderate physical activity may enhance immune function. Still, excessive amounts of prolonged, high-intensity exercise may impair the immune system, increasing the risk of upper respiratory tract infections, especially among athletes [10]. That correspondence is known as the exercise paradox phenomenon, and it has been modeled in the form of a J-shaped curve. Also, it was reported that obesity, emotional stress, and cigarette smoking had been incriminated for a decreased immunity, enhancing viral infections [11]. However, no studies determine the role of physical exercise and other lifestyle habits as a serious predisposing factor for viral myocarditis infection.
Therefore, this study aimed to investigate if specific lifestyle risk factors such as smoking, body weight, emotional stress, and physical inactivity among young adults are related to viral infection leading to myocarditis.
The study evaluated forty (40) young adults, 36 men and four women, aged 18-45 years old, diagnosed and hospitalized with viral myocarditis for the last five years (Group A) in two general hospitals of Thessaloniki (ΑHΕPΑ Hospital and 424 General Military Hospitals). The diagnosis was set according to the medical history, the ECG, troponin levels, and clinical signs and symptoms and confirmed by cardiac magnetic resonance imaging (MRI). The control group (Group B) consisted of forty aged-matched adults, 28 men and 12 women, aged 19-47 years old, who entered the hospital with symptoms attributed to acute myocarditis, but the diagnosis was not confirmed. The Ethics Committee of the School of Physical Education and Sport Science at Thessaloniki approves the study's design (Approval number 84/2021).
Patients with coronary heart disease, hypertension, diabetes mellitus, and other chronic diseases were excluded from both groups. The study was performed in the pre-COVID 19 eras.
The parameters that were considered in all patients were
Statistical tests were performed using the SPSS version 25.0. Continuous variables were reported as mean ± SD, with categorical variables as counts and percentages. Continuous data were compared between groups using a two-tailed unpaired Student t-test if variables are normally distributed and a MannWhitney U test if not. As appropriate, discrete variables were compared using Fisher's exact probability test due to the smallsized sample.
Multiple logistic regression analyses with the "enter" method were performed to examine relations between myocarditis and other variables. A 2-tailed P value < 0.05 was considered statistically significant.
Drugs taken by the patients, also lipid-lowering drugs (statins, ezetimibe, PCSK9 inhibitors), were continuously recorded.
All patients gave their written informed consent to this evaluation which had been approved by the local ethics committee (EK 3222082014).
Patients with myocarditis (Group A) mentioned previous respiratory tract infections (50%), gastrointestinal infections (20%), and sinuses and throat infections like the common cold (17%). All patients were referred to thoracic pain. Dyspnea was mentioned in 21 of them. ST abnormalities in the ECG, such as depression or elevation, were observed in 38 patients, and arrhythmias in 8. Troponin levels were remarkably high in all patients. Finally, the diagnosis was confirmed by MRI (Fig. 1). During the first year, follow-up of the patients didn't show disease progression or deterioration.
All patients in the control (Group B) mentioned thoracic pain. Dyspnea was referred from 28 of them. Twelve have ST abnormalities in their ECG and fourteen high troponin levels. The results of the MRI were negative for myocarditis. Five patients underwent coronary angiograms, which established coronary disease. Finally, eight controls were diagnosed with gastroesophageal reflux, three acute pericarditis, four pneumonia, four acute bronchitis, five influenza-like symptoms, and seven musculoskeletal disorders, while four of the cause remained undiagnosed.
Levels of Physical Activity and Myocarditis
Thirty patients in Group A (75%) and thirty-six in controls (90%) reported low activity levels (p<0,005). Five (12. 5%) in group A and 4 (10%) in group B trained at a moderate rate (NS). On the other hand, high or very high physical activity levels were reported in only 5 (12.5%) in group A and none in group B, respectively. Therefore, significant differences (p: 0.006) were found between the two groups regarding the low and high levels of physical activities.
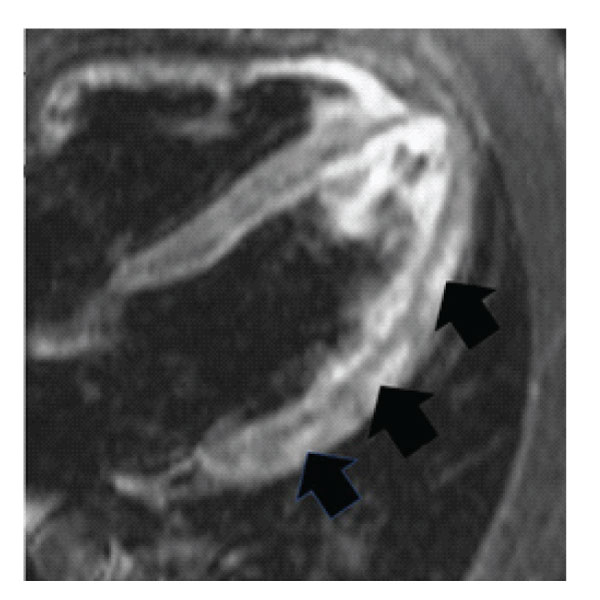
Figure 1: IMR imaging of 28 years old patient with acute myocarditis (arrows). The patient was a heavy smoker.
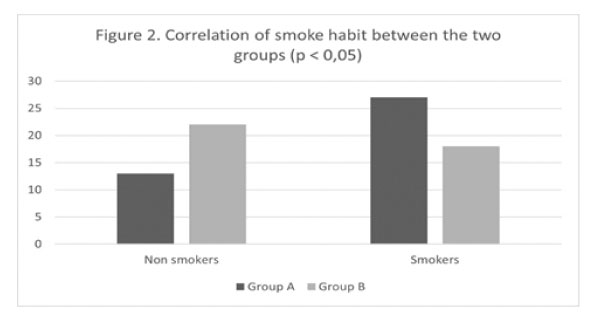
Figure 2: Correlation of smoke habit between the two groups (p<0.05).
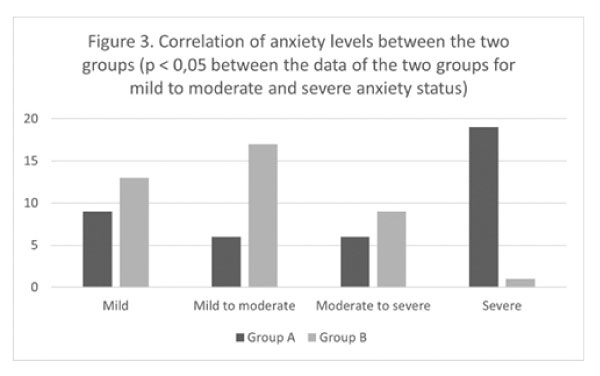
Figure 3: Correlation of anxiety levels between the two groups (p<0.05 between the data of the two groups for mild to moderate and severe anxiety status).
Chi-square analysis comparing different physical activity levels in the myocarditis infection group (group A) was studied and revealed a statistically significant relation. Specifically, 53.8% of patients with deficient level activity (almost sedentary life) were diagnosed with myocarditis (28 patients). The 14.3% of patients (2 patients) had low physical activity. 55.6% (5 patients) had moderate activity, while the 100% of patients had high (1 patient) and very high levels of activity (4 patients who were active athletes with systematic strenuous training).
Smoking and Myocarditis
The percentage of smokers was higher than that of nonsmokers (Fig. 2). Forty-five patients in group A and controls (56.3%) were characterized as "smokers." Specifically, in group A, 27 of the patients (67.5%) were smokers, while in group B, 18 were smokers (45%). There was a significant difference (p: 0,043) regarding "smoking" between the two groups. Statistical analysis comparing smoking and myocarditis infection were revealed a significant (p<0.005) correlation.
BMI and Myocarditis
Most of the patients with myocarditis and controls (65%) had body mass index within the normal range. Thirteen patients (32.5%) in group A and 14 (35%) in group B were overweight. There were no significant differences between the two groups (p: 0,815). There was also a negligible percentage of obese (1.3%).
Level of Stress
The level of stress was characterized as severe in 19 (47.5%) of the patients in group A and only in one patient in group B (p<0.005Fig. 3). Mild to moderate stress was reported in 15 (37.5%) of the patients in group A and 30 (75%) in group B (p<0.005). Moderate to severe stress was recorded in 6 (15%) patients in group A and 9 (22.5%) in group B (NS). Statistical analysis comparing intense anxiety level and myocarditis infection were revealed a significant (p<0.005) correlation.
Multivariate Analysis
The multivariate analysis found that the statistically essential factors for myocarditis are stress (p: 0,002), which multiplies x 5 times the risk of infection, and smoking bears x3 times the risk (p: 0,027). The result is adjusted for the levels of activity, BMI, smoking, and stress (Table 1).
It was reported that most patients with viral myocarditis were young and healthy previously [18]. However, some individuals are more susceptible such as pregnant women and those who are immunocompromised. No references exist for the impact of common risk factors on the appearance of viral myocarditis. An essential finding of the study is that a sinful lifestyle, especially smoking and stress can be significantly blamed for the more frequent viral infections causing acute myocarditis.
Table 1: Multivariate analysis of risk factors for myocarditis (Group A).
Variables |
Exp(B) |
CI(Lower) |
CI(Upper ) |
P |
Physical activity |
2,082 |
0,496 |
8,739 |
0,316 |
BMI |
0,643 |
0,221 |
1,874 |
0,418 |
Anxiety |
5,169 |
1,803 |
14,824 |
0,002 |
Smoking |
3,174 |
1,137 |
8,865 |
0,027 |
The responsible viruses must have a specific mechanism to induce myocarditis. For example, the Coxsackie B3 virus produces 2A protease, dividing dystrophin in the cardiac membrane. Consequently, a functional disorder throughout the cardiac membrane leads to cell dysfunction [19]. Opavsky et al. [20] reported increased survival and decreased myocardial damage after viral infection in CD4−/−CD8−/− mice. Interestingly, viral myocardial clearance was not impaired in the CD4−/−CD8−/− mice compared to wild-type mice, suggesting that both CD4+and CD8+ T cells participate in cardiac tissue damage and do not influence viral titer. Although previous studies have focused on the critical role of acquired (adaptive) immunity in the pathogenesis of viral myocarditis [21], the innate immune system's role in viral myocarditis is less understood. The natural immune system plays an essential role as a primary sensor of invading pathogens and the induction of host antimicrobial defenses.
The present study results showed that people with reduced physical activity suffered more often from acute myocarditis compared to people with moderate or high physical activity in their lifetime. However, the controls appeared to have a more sedentary life than the myocarditis group. Because of the above relationship and accounting for the small number of patients participating in the study, it is impossible to draw firm conclusions about the impact of sedentary life in developing acute viral myocarditis. However, previous studies support that regular moderate exercise decreases the risk of upper respiratory viral infections due to establishing an anti-inflammatory environment [10,11,22]. Especially, mild to intermediate exercise training leads to a) increased plasma cortisol and adrenaline levels, b) increased IL-6 production by skeletal muscles, c) decreased TLRs expression in the monocyte cells and macrophages, d) decreased adipose tissue, e) changed the phenotype of macrophage cells into the fatty tissue, f) reduced the number of pro-inflammatory monocytes, g) increased the number of circulating Treg cells.
On the contrary, strenuous athletes seem to be vulnerable to common infections. This derangement may be due to increased anti-inflammatory cytokines IL-10 and other reported immunosuppressive affection of the heavy training [23,24]. Those results created the so-called "open-window" hypothesis, which purports that the immune system is compromised in the hours after vigorous exercise, leading to an increased risk of opportunistic infections in the days after that [11,24,25].
Contrary to the reports mentioned above that vigorous exercise heightens infection incidence, it is often overlooked that other studies indicate that every exercise participation may reduce the incidence of infections [26]. Therefore, controversial evidence supports the claim that heavy exercise suppresses cellular or soluble immune competency.
Smoking is a substantial risk factor for viral infections, especially upper respiratory infections, inducing myocarditis. Mechanisms by which smoking increases the risk of infections include structural changes in the respiratory tract and decreased immune response [27,28]. Our study found a significant positive correlation between smoking and acute myocarditis since the majority (60%) of smokers were diagnosed with myocarditis. In addition, smoking impacts innate and adaptive immunity and plays dual roles in regulating immunity by either exacerbating pathogenic immune responses or attenuating defensive immunity [29]. Adaptive immune cells affected by smoking mainly include T helper cells (Th1/Th2/Th17), CD4+CD25+ regulatory T cells, CD8+ T cells, B cells, and memory T/B lymphocytes. In contrast, innate immune cells impacted by smoking are mostly DCs, macrophages, and NK cells.
It was supported that obesity has been associated with respiratory infection risk [30,31]. Paradoxically the association with obesity was confined to those physically more active and not seen for those reporting little sports activity, possibly due to the oxidative stress after the exercise [30]. There is no obese patient among the forty patients with myocarditis in our study. Moreover, no association existed between BMI and viral myocarditis. Obesity, like other states of malnutrition, is known to impair immune function, altering leukocyte counts and cell-mediated immune responses [31]. Besides, evidence has arisen that an altered immune function contributes to the pathogenesis of obesity. Innate and adaptive immune cell-derived inflammation drives locally and systemically insulin resistance [32]. However, new evidence suggests that the immune system is vital for adipocyte differentiation and protection from ectopic lipid deposition [33]. Furthermore, the roles of anti-inflammatory, immune cells such as regulatory T cells, "M2-like" macrophages, eosinophils, and mast cells are being explored, primarily due to the promise of immunotherapeutic applications [30,31].
The outcomes in the present study revealed a statistically significant association between stress levels and myocarditis; 95% of patients who reported high-stress levels were diagnosed with myocarditis. Therefore, we suggested that high-stress levels increased the risk of myocarditis after a viral infection. Stateanxiety and perceived psychological stress levels before activity are essential in determining the in vivo immune response's strength after exercise [11,34]. Furthermore, stress is associated in a dose-response manner with an increased risk of acute infectious respiratory illnesses [16,17].
Many neurobiological processes related to stress and depression have been observed in anxiety and influence the immune system. A review of the immune response to stress and immune alterations in depression has been presented further to understand the biology of anxiety [35]. It appears that a variety of factors such as age; sex; nature, intensity, chronicity of stressful life events; and psychological response to life stress need to be considered in the investigation of behavior and immunity. Thus, the biological effects of stress on immunity are multifaceted, including complex neuroendocrine and neurotransmitter interactions [17].
When interpreting our results, some limitations should be taken into consideration. First, the sample is limited by its size. Some groups included very few patients; therefore, it was arbitrary to draw certain conclusions. Secondly, the combined presence of risk factors in a patient (e.g. smoking and stress) was not statistically analyzed. Finally, the viral origin of myocarditis was based on the clinical picture and history and was not identified in all using specific serological reactions. These limitations restricted the validation of the study.
In conclusion, the study suggests an association between some lifestyle habits and viral infection risk leading to myocarditis. Specifically, high-stress levels, cigarette smoking, and possibly a sedentary life could be associated with an elevated prevalence of acute myocarditis due to potentially adverse effects on the immune system. However, future studies are needed to confirm the results and examine the potential biological mechanisms.