We wish to report a very rare and interesting case of a 3-year-old toddler boy who presented with request for cultural circumcision. On physical examination, the distal shaft of penis and glans area was swollen and the foreskin was initially non-retractile but with gentle persistent retraction, a large hidden cavernous venous vascular malformation (CVVM) of the glans penis and distal portion of the shaft of the penis was evident. Ultrasound and Color Doppler confirmed low flow CVVM. Penile magnetic resonance imaging (MRI) showed a well-defined vascular lesion in the form of a CVVM of the glans and the distal half of the penile shaft in the subcutaneous area measuring 17 X 23 X 36 mm with a small polypoid external component and extending to the corpora spongiosum and cavernosa. The patient underwent high dose short term steroid therapy for 5 weeks with near complete resolution of the lesion. The patient underwent cultural circumcision subsequently which led to the complete resolution of the residual lesions and acted as supplemental form of therapy. Patient is asymptomatic and well at 4 years follow up.
Keywords: Congenital Vascular Anomalies; Cavernous Venous Vascular Malformation; Circumcision; Glans Penis; Hemangioma; Penis; Steroids.
Abbreviations:
CVVM- Cavernous venous vascular malformation.
MRI- Magnetic Resonance Imaging (traditional sequences-T1 and T2, Specialised sequence-STIR).
T1 Images – 1 tissue type is bright – FAT.
T2 Images – 2 tissue types are bright – FAT and WATER.
STIR - Short Tau Inversion Recovery- images are highly water-sensitive and the timing of the pulse sequence used acts to suppress signal coming from fatty tissues – so ONLY WATER is bright.
ISSVA- International Society for the Study of Vascular Anomalies.
GERD-Gastro-Esophageal Reflux Disease.
Vascular anomalies represent a spectrum of disorders from a simple "birthmark" to life- threatening entities. Congenital lesions of the glans penis and penile shaft are very rare [1,2]. Congenital vascular anomalies are an all-inclusive term for the two “simple” forms that is vascular malformations and vascular tumors in the form of hemangiomas, “combined” malformations, other “complex” syndromic and “indetermined/unclassified” congenital vascular defects [3,4]. Congenital vascular tumors or hemangiomas are the commonest vascular anomalies in infants and vascular malformations are seen occasionally in older children and adults as they appear externally later in the life [5,6,7,8]. Only 2% of vascular malformations occur in the urinary tract. Even among urinary tract vascular malformations, cases where it occurs on the glans penis are extremely rare. Vascular malformation is a benign vascular lesion. Cavernous and mixed varieties are most common and mostly found in the musculoskeletal system, liver, and spleen. It is rarely found in genitalia. CVVM of the glans penis is even a rarer lesion, and only 21 cases are reported in the literature [9]. We wish to present a case of CVVM of the glans penis and distal half of the penis in a 3-year-old boy with an excellent cosmetic and functional outcome by using steroids followed by circumcision.
An otherwise healthy 3-year-old boy presented with request for cultural circumcision. The parents have first noticed an irregular fullness on the ventral aspects of the distal penile shaft and glans penis at the age of 2 weeks which increased in size for few months. There was no other lesion in the rest of the body. There was no history of trauma, infection, ulceration or bleeding from the lesion.
Physical examination showed vital signs within normal limits. On local examination, the distal shaft of penis and glans area had elevated irregular lesion. The foreskin was initially non-retractile but with gentle persistent retraction, a large hidden CVVM was evident on the glans penis and distal shaft (Fig. 1A). It was bluish-pink in color with a smooth surface and irregular margin, compressible, nontender, and non-pulsatile.
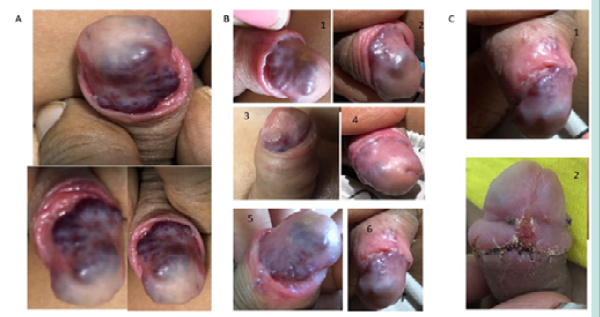
Figure 1: A) lesion at presentation- before intervention and B) Resolving lesion with steroid therapy-initial and weekly photos and C) at follow up 3 months and post circumcision appearance.
Color Doppler ultrasonography confirmed the low flow state in the lesion consistent with the diagnosis of CVVM. Abdominopelvic ultrasound was normal. Penile MRI using T1, T2 and Short Tau Inversion Recovery (STIR) weighted sequences with dynamic contrast was carried out. It showed well defined vascular lesion in the form of CVVM with abnormal hyperdense signal in the subcutaneous region in the distal half segment of the penis and glans with intense post contrast enhancement and a small external polypoid component measuring 17 X 23 X 36 mm and extending to the corpora spongiosum and cavernosa (Fig. 2).
We have explained the final diagnosis and given an overview of all available options in the treatments with our preference for the medical treatment initially. The use of propranolol and prednisolone for the treatment of hemangioma and vascular malformations have become hugely popular and propranolol is preferred for the hemangiomas in infants with proliferative phase due to its effectiveness and safety profile. The oral propranolol may be started at 1 mg/kg/day and titrated to 2 mg/kg/day with pulse rate and blood pressure monitoring for an intended duration of 12 months according to the Great Ormond Street Hospital protocol. However, treatment failures at this age with vascular malformation were very likely, it was difficult for parents to administer medication that long in a toddler and their local primary care family physician and pediatrician were not very familiar with this treatment and hence it was not instituted and steroid therapy was selected.
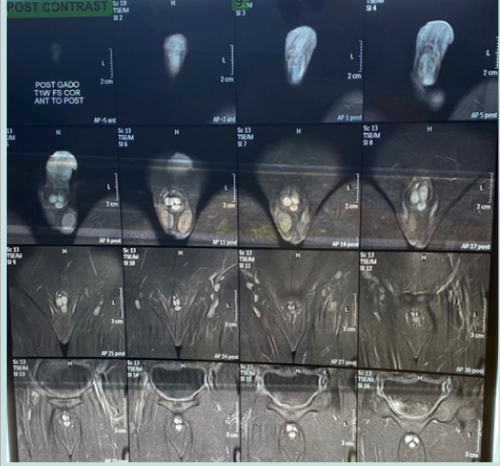
Figure 2: Post contrast images of MRI with T1 images showing no fat, T2 images showing fluid and specialised STIR sequence highlighting water only in the gals and penile lesions.
Prednisolone was started at 0.3mg/kg/day and titrated to maximum dose of 3 mg/kg/day for 5 weeks in accordance to findings from a systematic review for the treatment of cutaneous hemangiomas [3,4]. The lesion started showing resolution while on therapy as shown in the weekly photographs (Fig. 1B). At 3 month follow up, near complete resolution with some residual small lesions were noted (Fig. 1C1).
The patient underwent examination under anesthesia and circumcision uneventfully (Fig. 1C2). The excised foreskin histology showed microthrombi in the blood vessels and scar tissue and involuting some elements of CVVM in subcutaneous tissue at the base and no active proliferative lesion. The patient is asymptomatic and doing well at 4 years follow up without any rebound or recurrence on Ultrasound and Colour Doppler with residual umbilicated scar on the glans, well healed circumcision scar and wrinkled distal penile shaft skin with prominent subcutaneous veins as seen in (Fig. 3).
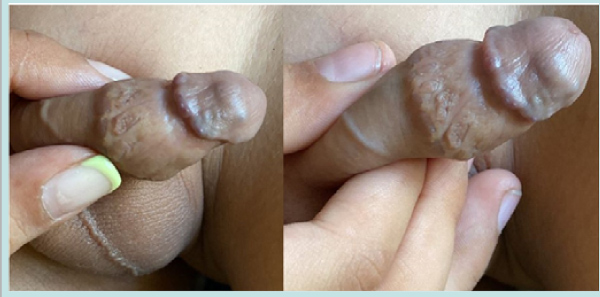
Figure 3: Long term follow up full and close up views-note the umbilicated residual scar of resolution on the glans, well healed circumcision scar and disappearance of distal penile swelling with wrinkled skin and prominent subcutaneous veins.
Hemangiomas have plump endothelia, increased mast cells, and multi-laminated basement membranes and are superficial, deep or combined and may be proliferating or involuting. Infantile hemangiomas are not inherited but by mutation in a primitive stem cell responsible for developing blood vessels, is a model of pure, unopposed angiogenesis with common expression of immunohistochemical markers including glut-1, Fcy RII, Lewis Y antigen, etc., has possible derivation from or sharing a common precursor with placenta and angiogenic peptides. The markers involved in the proliferating phase – expression of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and type IV collagenase while in the involution phase – tissue inhibitor of metallo-proteinases (TIMP-I) and mast cell secreted modulators.[3].
Vascular malformations have flat endothelia, normal mast cell numbers, and a thin basement membrane and may be classified into capillary, venous(cavernous), arteriovenous, arterial, lymphatic and mixed subtypes with a combination of these. Vascular malformations are usually sporadic but can also be inherited in a family as an autosomal dominant trait with variable expression and incomplete penetrance. They are a manifestation of many different genetic syndromes that have a variety of inheritance patterns and chances for reoccurrence, depending on the specific syndrome It has been estimated that up to 60% of all vascular anomalies are misdiagnosed. In our search for literature even the most recent case has been reported is as hemangioma of glans and penis but in fact they are all cases of vascular malformationsas discussed later [4].
Classification schemes provide a consistent terminology and serve as a guide for pathologists, clinicians, and researchers. We have some comments about the terminology and the classification. According to the International Society for the Study of Vascular Anomalies (ISSVA) classification of vascular anomalies, there is a clear distinction between the two simple vascular anomaliesvascular tumors (hemangiomas) and vascular malformations [10]. While reviewing the literature, we observed that the most authors seem to mix congenital hemangiomas, infantile hemangiomas and vascular malformations, as for the diagnosis and treatment modalities [11,12,13,14,15]. "Cavernous hemangioma" is an old terminology of a vascular anomaly made by low flow dysplastic chambers containing venous blood, corresponding to a "venous malformation" in the new classification [10]. Since the publication of the seminal work on the histology-based classification of vascular anomalies by Mulliken and Glowacki in 1982 and the subsequent adoption of an expanded and modified version in 1996 by the International Society for the Study of Vascular Anomalies, an increasing number of vascular lesions have been recognized as histologically distinct entities [16]. Hence, we have changed it to CVVM rather than hemangioma as per the most recent calcification of these congenital vascular anomalies.
With regards to the molecular and genetic basis of the congenital vascular anomalies in general and that of the CVVM in particular, genes mutated in CVVM encode proteins that modulate junction formation between vascular endothelial cells. Mutations lead to the development of abnormal vascular structures characterized by thin-walled, dilated blood vessels with gaps between the endothelial cells. The underlying genetic mechanism in CVVM is partially understood. At least three genes have been associated with CCM: k-rev interaction trapped protein 1 (KRIT1) (CCM1; OMIM no. 604214), MGC4607 (CCM2; OMIM no. 603284) and programmed cell death 10 (PDCD10) (CCM3; OMIM no. 603285) [17,18].
Accurate diagnosis is crucial for appropriate evaluation and management, although both types of simple vascular anomalies may appear similar apparently; there are several differences between them [3]. In fact, hemangiomas and vascular malformations have nothing in common except sometimes a somewhat similar appearance [4]. Hemangioma is a solid benign vascular tumor; only occur in infants appearing after 2 weeks of age; has female preponderance with ratio of F: M::5:1; has proliferative endothelium with cellular hyperplasia; associated with variety of appearances such as -raised, flat, smooth, bosselated, superficial, deep-blue subcutaneous; grows fast in the first 9 months and slowly involutes and Glut +ve; 80% in head & neck, outside/inside body- e.g., brain, liver, intestine; feels spongy, warm, compressible, rapid refill, pulsatile having bruit; may be complicated by ulceration, infection, bleeding; treatment straight forward and conservative or medical therapy; and prognosis generally good [4]. While the vascular malformation can occur at any age; equal in both sexes; have defects in the vascular morphogenesis; appear as thin & deep, diffuse, focal or fruit like, infiltrative & destructive; usually superficial ones are visible & present at birth; most grow slowly and none involute; can be in brain, liver, intestines, spine, stomach, or organs; complicate with bleeding; lymphatic swell/shrink with respiratory movements, venous fill when dependent, arterio-venous have pulse when pressed and transilluminate; treatment complex, multidisciplinary-multimodality and prognosis variable [3].
Ultrasound with Color Doppler will help to demonstrate low flow state. However, imaging (particularly MRI) is needed in most symptomatic patients to confirm the suspected diagnosis and to evaluate the extent of the lesion. In addition to making the diagnosis, MRI plays a major role in decision making regarding how these lesions need to be treated-surgery versus embolization/ sclerotherapy [3]. In the MRI, T1 and T2 sequences are well known to all of us but Short Tau Inversion Recovery (STIR) sequence is used for water containing lesions such as cavernous vascular lesions. STIR images are highly water-sensitive and the timing of the pulse sequence used acts to suppress signal coming from fatty tissues – so ONLY WATER is bright. A combination of standard T1 images and STIR images can be compared to determine the amount of fat or water within a body part. The STIR sequence, designed to suppress signal from fat, also enhances the signal from tissue with long T1 and T2 relaxation times, such as neoplastic and inflammatory tissue. T2-weighted short-tau inversion recovery (T2w-STIR) imaging is the best approach for oedema-weighted cardiac magnetic resonance imaging (MRI), as it suppresses the signal from flowing blood and from fat and enhances sensitivity to tissue fluid. The most common MRI sequences are T1-weighted and T2-weighted scans. T1-weighted images are produced by using short TE and TR times. The contrast and brightness of the image are predominately determined by T1 properties of tissue. Conversely, T2-weighted images are produced by using longer TE and TR times [4].
One is in a dilemma to treat such lesion as there are several methods to treat and the aim is to treat it with minimum or no risk and object is to obtain maximum or best cosmetic and functional outcome. The treatment depends upon their size, location, and severity. There are a variety of more traditional methods of treatment in the form of observation (for the small, non-invasive lesions with possible expectation of nature cure with time), medical treatment with propranolol and systemic steroids (for moderate asymptomatic uncomplicated cases where cosmesis is more important), intralesional steroids, sclerotherapy (sodium tetradecyl sulfate,30% hypertonic saline and 2% polidocanol-invasive with attendant morbidity), vincristine, bleomycin (antiangiogenic drugs) or immunomodulation with interferon (in selected cases). Laser, electrofulguration, cryotherapy, interventional embolization. and surgical excision are more invasive and associated with higher risks are now reserved for unresponsive, acutely life-threatening, internal or that causes bleeding problems, feeding or breathing difficulties, growth disturbances or impairment of vision [3,4]. The medical treatment with propranolol and prednisolone is very safe, effective, can be treated on outpatient basis, simple, low cost and gives excellent cosmetic and functional effects with improvement or complete resolution of symptoms and relief from the potential complications.
The dose of prednisolone and its good effects for the treatment of infantile hemangioma is well documented in pediatric cases. In our case, we have titrated prednisolone to a maximum dose of 3 mg/kg/day in accordance to findings from a systematic review for the treatment of cutaneous vascular malformations [3]. This review found that prednisolone dose of 2 to 3 mg/kg/day was effective in 75% of cases with side effects reported in 16%. Whereas a dose of >3 mg/kg/day showed a 94% response rate with greater side effects reported at 51%. Less than 2 mg/kg/day of prednisolone was associated with fewer responses and adverse effects, but a rebound rate of 70%. The propranolol has less effects in older children with vascular malformations as compared to hemangiomas associated with higher failure rates and the side effects of hypoglycemia, sleepiness, GERD worsening, Irritable airways and bradycardia needs monitoring makes it a second choice as opposed to treatment of choice in young infants with proliferating lesions.
Concurrent use of systemic steroids at the time of propranolol initiation has been suggested to decrease the efficacy of propranolol therapy. However, this data was drawn from children who had concurrent propranolol and steroids at the initial treatment. More research is still needed to understand the interaction between systemic propranolol and steroid for the treatment of penile hemangioma. Hemangioma of glans penis is difficult to diagnose as it is covered with foreskin and its incidental detection after circumcision has been reported recently [15]. Early diagnosis would have detected smaller lesions and the effects of steroid treatment may be more pronounced with perhaps lower doses and shorter treatment duration. We believe that the circumcision following steroid treatment had supplementary additional therapeutic role and contributed to final resolution from near complete resolution by the steroid therapy. It may be because of the inflammation associated thrombosis of the feeding vessels in these venous cavernous lesions in the post-operative period and scarring associated with the healing process leading to a small residual scar at the sites of the clinical lesions.
In conclusion. prenatal diagnosis of the CVVM of glans penis and penile shaft may be difficult as most lesion develop after birth in infancy or later on but at neonatal or infant examination, high index of suspicion for symptomatic congenital lesions and fullness or swelling of the distal shaft and glans penis should prompt for an ultrasound with color doppler at birth or in early infancy when first noted. In general, early diagnosis of this lesion is key to successful medical treatment. Propranolol is the treatment of choice in hemangioma and early commencement of propranolol is necessary to achieve maximal improvement in symptoms and prevent further proliferation. This will allow early treatment, giving the best chance to avoid treatment failure or propranolol resistance in older children as has been reported in several cases and in such cases of vascular malformation treatment of choice would be steroid therapy with excellent cosmetic and functional results. The circumcision following steroid therapy seemed to supplement the therapy with complete resolution from near complete resolution by the steroids complementing it perhaps with microthrombus formation and subsequent scarring postoperatively.
We are grateful to Dr Jitendra G Govani, Primary Care Physician and Dr. Dineshkumar L Kalaria MD, DCH consultant pediatrician for referring the patient to us and monitoring the systemic steroid therapy.
The authors have no conflict of interest to declare. No funding source was involved in this study
All procedures performed on human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent was obtained from the parents and all the relatives involved prior to all the procedures. Parents and all involved parties were informed about the procedure.