The present study aims primarily for the production of therapeutic bioactive compounds from Lacticaseibacillus rhamnosus KC6, a probiotic lactic acid bacterium (LAB) isolated from Curd. The main purpose to evaluate the therapeutic attributes was achieved via metabolic profiling of the isolate and in total, 13 metabolites were analyzed which are quite advantageous to humans, thus predicting the use of KC6 as a therapeutic agent. Furthermore, keeping the ongoing pandemic in view, we have designed the study in relevance with essential probiotic properties of the strain which might help reducing the negative impact of viral infection of COVID-19, thereby enhancing the immune system. So, various assessments were conducted including antimicrobial activity against clinically important pathogens and the isolate exhibited remarkable results as inhibition zones of 27mm>26mm>25mm>22mm for Clostridium perfringens, Escherichia coli, Staphylococcus aureus and Bacillus cereus respectively; while under few therapeutic properties, it displayed 87.17% of antioxidant activity; 60.33% lipid peroxidation inhibition activity and 77.72% cholesterol lowering activity. Alongside, we’ve testified various in vitro probiotic attributes including the tolerance as well as microbial adhesion, displacement and exclusion assays for which the overall outcome was 82.5%. Also, the safety assessment involving important tests for DNase, gelatinase and hemolytic activities were carried out which came out to be negative in each case, hence depicting its safer use. All these according to the guidelines of Joint FAO/WHO demonstrated the safety of strain and also revealed a noteworthy evidence of harboring strain-specific probiotic attributes which helped in the prediction of host in vivo probiotic beneficence and conclusively indicated that KC6 is highly capable of providing significant benefits to human host. In nutshell, the study affirms the use of L. rhamnosus KC6 in development of therapeutic compounds as well as in formulation of new functional foods in future for the betterment of human health.
Keywords: Therapeutic; Probiotic; COVID-19; Immunomodulatory; Antimicrobial; Antioxidant; Functional Foods.
Probiotics represent the “nonpathogenic living microorganisms which intend to provide quite a lot of health benefits to humans when consumed in appropriate amounts” [1]. These generally include Lactobacillus, Lactococcus, Enterococcus, Pediococcus, and Streptococcus genera of bacteria, which are generally found in fermented food matrices. Most bacteria used as probiotics belong to the LAB species, genus Lactobacillus (Lactobacillus acidophilus, Lacticaseibacillus casei, Lactobacillus crispatus, Limosilactobacillus fermentum, Lactobacillus gasseri, Lactobacillus helveticus, Lactococcus lactis, Lactiplantibacillus plantarum, Limosilactobacillus reuteri and Lactocaseibacillus rhamnosus) and genus Enterococcus (Enterococcus faecalis as well as Enterococcus faecium). Because LAB starter cultures have been used to carry out food and milk fermentation since decades, these bacteria are widely regarded as 'generally recognised as safe' (GRAS) [2,3]. The majority of Lactobacillaceae sp. strains play a role in protecting the gastrointestinal mucosa from pathogenic strains like E. coli, S. typhimurium, and E. faecalis. Lactobacillaceae strain adherence and competitive adherence to cell lines have been studied, indicating that these strains have a protective role. Similarly, bacterial auto-aggregation and co-aggregation may lower the degree of pathogenic strain colonisation of the mucosa [4]. That is why, probiotic therapy is said to be eminent in curing various lifestyle related disorders which are quite frequent these days. Sundry of probiotics have distinct potential to confer different advantageous effects over the living system. Nutrition is an essential regulator of the immune homeostasis, and even small deficiencies of some micronutrients could disturb the immune response. Customarily prepared cuisines usually include various fermented foods, as these bacteria reinstate the balance of microbes within the human gut [5]. Explicitly, in probiotic research field several remarkable advancements have been observed in the preceding few decades which allow more inventive generation of plentiful persuading data based on the functionality as well as mode of action of these probiotics, taking account of their beneficial effects over the gut-microbiome along with their eventual outcomes over the clinical health as well [6]. Several clinical trials have also provided the evidence of strainspecific probiotics which are beneficial for a lot of health issues. Throughout the research history of past two decades, many studies along with the clinical trials have conjured up the beneficence of probiotics especially in modulation of immune responses along with the treatment of various disorders, exclusively those involving viral infections, especially COVID-19 [7]. Several findings have also demonstrated that probiotics efficiently maintain host’s healthy immune system, thus helping the body to ricochet after the attack of virus on the respiratory tract in animal models. Along with these interpolations also intend to drop down the viral load within lungs and boost the patient’s survival rates by enhancing the health [8]. Hence, with an adequate supplementation of a suitable probiotic, human health can be augmented. Table 1 portrays a rough list of probiotic strains whose beneficences have been reported in various recent studies in prevention of infections as well as enhancement of immune functions in order to reduce the adverse effects of viral infections [19].
Table 1: List of probiotic strains and their beneficences in prevention of infections and immune function enhancement.
Probiotic strains |
Study |
Conclusions |
References |
Lacticaseibacillus rhamnosus HN001, Lactobacillus acidophilus DDS-1, Bifidobacterium lactis Bb-12, and Streptococcus thermophilus |
Upper respiratory tract infection in 6269 participants of a clinical trials |
A meta-analysis of 23 trials reported that consumption of probiotics reduced the prevalence of respiratory tract infections and also the improved quality of life |
|
Enterococcus faecalis |
Influenza virus (A/WSN/33) and Enterovirus 71 in male C57BL/6 mice |
Improved survival rate as well as reduced viral load in the bronchoalveolar lavage of the infected mice |
|
Lactobacillus acidophilus, Lactobacillus reuteri, and Lactobacillus salivarius |
Influenza virus A/Duck/Czech/56 (H4N6) in Madin-Darby canine kidney cells |
Enhanced antiviral activity of chicken macrophages. Significantly higher expression of IL-1β, IFN-γ, and IFN-α resulted in protective responses against infection |
|
Lactobacillus paracasei N1115 |
Upper respiratory tract infection in 233 participants of a clinical trial |
Reduced provenance of upper respiratory tract infections along with a higher percentage of CD3+ cells |
|
Lactobacillus casei strain Shirota |
Upper respiratory tract infection in 96 participants of a clinical trial |
Healthy subjects reported an expressively lower (22.4%) incidence of acute respiratory infections than 53.2% in case of control group. |
|
Lactobacillus paracasei, Lactobacillus casei 431, and Lactobacillus fermentum PCC |
Upper respiratory tract infection in 136 participants of a clinical trial |
50% to 60% reduced prevalence of common cold and flu-like symptoms and increased levels of IFN-γ and IgA |
|
Lacticaseibacillus rhamnosus GG, Lactobacillus reuteri, and Bifidobacterium infantis 35624 |
Multiple diseases in a course of meta-analysis of 52 clinical trials |
Probiotics were most effective against the acute respiratory tract infections, acute infectious diarrhea, antibiotic-associated diarrhea, infant colic as well as necrotizing enterocolitis. |
|
Bifidobacterium bifidum |
Influenza virus-A/PR/8/34 (H1N1) in female BALB/c mice |
Increased survival rate along with the induction of both humoral and cellular immune responses |
|
Lactobacillus gasseri SBT2055 |
Respiratory syncytial virus-A2 strain in female BALB/c mice |
Reduced weight loss, lower viral load in the lungs of infected mice along with the reduced expression of proinflammatory cytokines |
|
Lacticaseibacillus rhamnosus GG, Lactobacillus casei, Bifidobacterium lactis Bb-12 |
Acute respiratory tract infections as well as acute otitis in ameta-analysis of 17 trials |
Probiotic strains significantly lowered the prevalence of such common acute infections along with antibiotics utilization |
The novel coronavirus infection i.e., COVID-19 which has been recklessly hiking since past two years on the entire planet has raised a significant concern of the researchers over the matter of eradicating [10]. The transmission of SARS-CoV-2 generally occurs through the respiratory droplets while now is likely to be transmitted through aerosols as well. It harbors the spike proteins which mediate the viral adherence with the receptor of host cell namely; angiotensin-converting enzyme 2 i.e., ACE2 receptor. With the successful adherence the viral entry expedites which gradually leads to local inflammation and ultimate impairment of multiple organs [20]. The damage is often concomitant with oxidative stress resulting to severe damage to the cell membranes. The patients struggling with COVID-19 infection exhibit high intensities of inflammatory cytokines within the bloodstream, referred to as cytokine storm and this is the triggering reason for this acute multi-organ damage [19]. COVID-19 is also associated with the dysbiosis of gut which leads to escalation of pathogenic microbes in the host gut. This altogether along with extreme viral load in mucosa results in the disruption of the gut epithelium leading to a chronic illness. Probiotics may rectify the dysbiosis by diminishing the viral load as well as inflammation. Likewise, strengthening of the host immune system proves to be the most efficient way of lessening the severity of COVID-19 [21] and probiotics are likely to offer a beneficial and credible way for the accomplishment of this purpose. With the rising pandemic, an increased prominence over the general public health has incited over few years. In the present study as well, we have led the main focus upon the novel therapeutic compounds’ production as well as knowing and understanding various probiotic attributes of the LAB isolate Lacticaseibacillus rhamnosus KC6.
The probiotic isolate was isolated from a traditional fermented food product i.e., Curd of Trans Himalayan regions. The curd samples of curd locally called “Dahi” were collected from different parts of H.P and pooled together and stored in refrigerator before processing for isolation of LAB. Then 1 ml of pooled sample was added to 9 ml sterilized distilled water in order to make the stock solution followed by the serial dilution in 10-1 to 10-9 range. 0.1 ml of the sample from each dilution was then mounted by the spread plate method onto the sterilized petri dishes of solidified de Man, Rogosa, Sharpe (MRS) agar medium and which were then kept for incubation at 37oC for 48 h. After that, we selected and purified the individual colonies of LAB isolate via streak plate technique and the pure cultures were further kept for preservation on MRS slants having 40% glycerol inside a deep freezer (-20˚C) [22].
Production of Therapeutic Metabolites
Metabolic Fingerprinting
Extraction of the metabolites was done using the methodology given by Coucheney et al. (2008) [23] with slight modifications. Microbial suspensions (30 mL) of each isolate were cultivated in MRS broth for 24h at 35oC. Suspensions obtained were then brought for centrifugation at the g-force of 8,400×g for about 10 min in order to separate the intracellular metabolites from within the cells. Then the supernatant was eliminated and the extraction of metabolites was done with methanol: water: chloroform mixture in 2:0.8:1 ratio. 2.5mL of cold chloroform along with 5 mL of cold methanol (-20oC) were added to and the phases were allowed to separate. Profiles of metabolites were measured in aqueous phase after freeze drying while in the organic phase; the dried chemical extracts were obtained by complete evaporation of the solvent.
The dried chemical extracts were obtained by complete evaporation of the solvent. Further analysis and measurement of profiles of metabolites in aqueous phase after freeze drying while in the organic phase under specific instrumental conditions was done in outsource agency of NIPER, S.A.S Nagar, MohaliPunjab. TG 5MS (30m X 0.25mm, 0.25µm) column was used for the analysis. S/SL type injector adjusted to 250˚C temperature was used and 1.0 µL of sample was injected in one run. The temperature of MS transfer line was set 260˚C while that of the ion source was set 230˚C. Helium was the carrier gas which was run at 1 mL/min flow rate in the GCMS column. The detector used in this instrumentation was TSQ 8000 triple quadrupole MS.
Antagonistic Spectrum
Procurement of Indicator Bacteria
LAB isolate was tested for their antagonistic potential by well diffusion method against test indicators viz. Bacillus cereus CRI, Clostridium perfringenes MTCC 1739, Escherichia coli IGMC, and Staphylococcus aureus IGMC. All the indicators used to check the antagonistic activity of L. rhamnosus KC6 were maintained on nutrient agar slants at 4oC. All indicators were sub cultured periodically at 35oC.
Well Diffusion Method
L. rhamnosus KC6 was tested for its antibacterial activity via well diffusion method as given by Kimura et al. [24]. After that, activity units of the bacteriocin produced by the bacterium were calculated by measuring the clearance zones around the wells. Clear zones of inhibition obtained were observed critically and AU/mL was calculated as following:
Therapeutic Properties
Determination of Antioxidant Activity-DPPH Free Radical Scavenging Assay
The cell free extract of the bacterial isolate was prepared according the methodology described by Afify et al. [25] which was then subjected to DPPH free radical scavenging assay following the procedure described by Heo et al. [26] and the percentage of antioxidant activity was evaluated using to the following equation:
Where, AS: Absorbance of sample at 517nm
AC: Absorbance of control at 517nm
Lipid Peroxidation Inhibition Activity of Lab in a Liposome System
Inhibition of lipid peroxidation by LAB was detected by the 2-thiobarbituric acid (TBA) method according to Kong et al. [27] and the percentage of inhibition of liposome peroxidation was calculated as follow:
Where, As: Absorbance of samples at 532 nm
Ac: Absorbance of control at 532 nm
Determination of Cholesterol Lowering Property
The ability of isolates to assimilate cholesterol was determined by according to Liong and Shah [28] and the ability of bacterial strains to remove cholesterol from the media was calculated as percentage from the following equation:
Where, Ab: Absorbance of culture supernatant at 550 nm
Ac: Absorbance of the control at 550 nm
Probiotic Properties
Tolerance Assays
pH and Bile Salt Tolerance
Acid tolerance of L. rhamnosus KC6 was determined following the method of Gupta and Sharma [29] and the % survivability was calculated with the formula as follows:
For examining the bile salt tolerance, the method of Gupta and Sharma [29] was followed and the % survivability was calculated with the formula as follows:
Tolerance of LAB to a Simulated GI Tract
Preparation of Simulated GI Juices
First of all, preparation of simulated gastric and intestinal juices was done to impersonate human GI tract condition [30]. Then, pepsin was suspended within aseptic phosphate buffered saline (PBS, 0.2 M, pH 3.0) in order to achieve gastric juice simulation with 3 g/L of a final concentration. On the other hand, trypsin was suspended within aseptic PBS (0.2 M, pH 8.0) in order to intestinal juice simulation with 1 g/L of a final concentration. These pepsin and trypsin solutions had to be freshly prepared daily followed by sterilization using 0.22 µm membrane filter.
Transit tolerance
It is known that, human gastric juice pH of is 1.0 during fasting while it increases to 4.5 after having a meal. The digestion of food requires about 3 h wholly. So, we re-suspended the collected LAB cells in sterile saline (0.85% NaCl, w/v) and then inoculated into the simulated gastric juice for achieving a final cell number of up to 108 colony forming units (cfu)/mL. Then the mixture was kept for incubation for 0, 1, 2, or 3 h at 37oC. After that, 1 mL of the culture was relocated to 9 mL of the simulated intestinal juice and kept for incubation for 0, 2, 4, or 8 h at 37oC [30].
LAB Cells Survival Rate
The serial dilution of the strain was done in sterile saline by transferring 0.1 mL dilution into the MRS-agar. MRS agar plates were incubated anaerobically for 48 h at 37oC for determining the total viable count. Then the LAB survival rate in the simulated GI conditions was evaluated using the following equation [31]:
Where, Nt: Total viable count of LAB after treatment with simulated GI juices
N0: Total viable count of LAB before treatment
Auto-aggregation and Co-aggregation Assays
Auto-aggregation assay for L. rhamnosus KC6 was executed following the method given by Gupta and Sharma [29] and the auto-aggregation % was calculated as follows:
Where, At: Absorbance at time t = 1, 2, 3, 4 and 5 h, and A0: Absorbance at t = 0 h.
Co-aggregation assay of L. rhamnosus KC6 was executed following the method given by Gupta and Sharma [29] and the co-aggregation % was calculated according to Handley’s equation [32].
Where, Apath: Absorbance of pathogen
ALAB: Absorbance of LAB
Amix: Absorbance of mixture (pathogen and LAB)
Bacterial Adhesion Assays
Bacterial Adhesion to Hydrocarbons
Bacterial adhesion towards hydrocarbon test (BATH) was carried out to assess the CSH. The test of adhesion to hydrocarbons was implemented to screen the isolate for cell surface hydrophobicity. Microbial adhesion towards hydrocarbons (MATH) was determined using the methodology of Gupta and Sharma [29] with slight modifications. Briefly, the overnight grown bacterial culture was washed and then resuspended in 3-mL 0.1M PBS, pH 7.0 to an OD600 = 0.5 (A0). 3mL cell suspension was then kept in 1mL of hydrocarbons viz. xylene, chloroform and ethyl acetate 10 min, after which, the two-phase systems were mixed by gentle vertexing for about 2 min followed by their allowed to separation by incubation for 2 hrs at 37 °C. The aqueous phase was then measured at OD600 (A1). The % hydrophobicity was then calculated as follows:
Where, At: Absorbance at time t=2 h
A0: Absorbance at t =0 h
Bacterial Adhesion to Mucus
Firstly, mucin (Type III from porcine stomach; Sigma) was made in phosphate buffered saline (0.1M PBS) (8.0 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4.2H2O and 0.2 g KH2PO4 per 1000 mL of dH2O, pH 7.3) at 0.5 mg/mL Nunc Maxisorp 96-well microplate (Roskilde, Denmark) wells were layered with the protein solution (200 µL per well) and incubated at 4oC overnight. Washing with PBS was done thrice for about 5 min followed by adding up of PBS supplemented with 0.1% Tween 20 (PBST) in order to saturate the wells lacking protein coat. After an incubation of 1h at room temperature, wells were again washed with PBS. The bacterial strain was then cultivated in MRS broth for 24 h at 35oC after which, the cells were harvested by a course of centrifugation at the g-force of 8,400×g for 10 min at 4oC and then washed two times with PBS only. The OD600 was adjusted with PBS to 1.0 OD. Then the bacterial cells (150 µL) were added into the micro-titer plate wells which were previously coated with 200 µL of gastric mucin [33].
Unlike the volume of the added bacteria, a greater volume of mucus was utilized to avoid the stain’s contact with the polystyrene. Cells were kept to adhere for 1h at 35oC and washing was given to the wells for three times with 250 µL of PBST to remove the non-adhered cells. Now, the adhered cells were allowed to fix at higher temperature of 65oC for 45 min. Further, the crystal violet method was employed to evaluate the actual adherence ability of the isolate [34]. Stained mucus lacking cells was used as the negative control. Results were expressed by subtracting the value of absorbance for the negative control from the value of absorbance recorded for the sample under study.
Displacement and Exclusion Assay
The ability of L. rhamnosus KC6 to displace already adhered pathogens was assessed through the method described by Collado and his coworkers for microbial adhesion to mucin with few modifications. The pathogenic bacteria (B. cereus, C. perfringens and L. monocytogenes) were added into the wells containing mucus and given incubation for 1 h at 37oC. After the removal of the unbound pathogens, bacterial cells of lactic acid bacterium were added and the plates were incubated for a period of 1 h at 37oC. Then the percent displacement of pathogens was evaluated by calculating the difference between the adhesions before and after bacterial addition [35].
Determination of competitive exclusion by L. rhamnosus KC6 was done by following the same procedure for microbial adhesion to gastric mucin with slight modifications. LAB and pathogenic bacteria were added up simultaneously into the wells containing mucus and then incubated for a period of 1 h at 37oC. After washing and removing the unbound cells, degree of competition was observed. Then the total exclusion was evaluated by calculating the percentage of bound pathogens after mixing with probiotic strain relative to bound pathogens in the total absence of probiotic strain [35].
Safety Assessments
Enzyme Activity Assay
L. rhamnosus KC6 was examined for the production of DNase enzyme using the methodology of Gupta and Sharma [29]. The culture was spot inoculated on DNase agar medium (HiMedia) and incubated for 48h at 37°C. The recorded characteristic of DNase enzyme production against a dark blue background was the appearance of clear pinkish zone around the colony.
The LAB isolate L. rhamnosus KC6 was also examined for the production of Gelatinase enzyme by inoculating 20 μL of 12h old cultures on plates containing MRS agar medium supplemented with 3% gelatin and incubating at 37°C for 48h. The plates were then flooded with the solution of saturated ammonium sulphate. Recorded characteristic of gelatinase enzyme production was the clear zone appearance around the spot [36].
Hemolysis Assay
Hemolytic activity of L. rhamnosus KC6 was tested using sheep’s blood agar plates (containing 5% of defibrinated sheep’s blood), incubated at 30°C for 24 h. Recorded characteristic of hemolytic activity was partial hydrolysis of the red blood cells i.e., greening zone, clear zone or no reaction around the bacterial growth implicating α-hemolysis, β-hemolysis and γ-hemolysis respectively [37].
Antibiotic Susceptibility
Antibiotic-resistance of L. rhamnosus KC6 was tested with 24 h old active culture the isolate swabbed on the MRS agar plates. The antibiotic-impregnated discs (Hi-media, India) were seeded on the swabbed plates and then after 24 h of incubation at 37oC, the zones of growth inhibition were precisely measured to discover its sensitivity for them [38]. The antibiotics tested were amikacin (AK) [10mcg], erythromycin (E) [5mcg], amoxycillin (AMX) [10mcg], gentamicin (GEN) [10mcg], azythromycin (AZM) [15mcg], norfloxacin (NX) [10mcg], bacitracin (B) [10units], novobiocin (NV) [5mcg], cefpodoxime (CPD) [10mcg], ofloxacin (OF) [5mcg], cephalothin (CEP) [30mcg], oxytetracycline (O) [30mcg], chloramphenicol (C) [30mcg], penicillin-G (P) [2units], ciprofloxacin (CIP) [5mcg], polymyxin B (PB) [100units], clindamycin (CD) [2mcg] and vancomycin (VA) [10mcg].
Cumulative Probiotic Potential
The cumulative potential of the selected strain to be used as a probiotic was calculated by using standard scorecard according to the method of [39].
The potential probiotic LAB was isolated from “Curd” which were evaluated for studying its probiotic attributes along with the immunomodulatory and therapeutic effects which it confers to the consumer. The sample was collected from Solan district of Himachal Pradesh and the pure culture of the isolate obtained was further subjected to the following experimental and analytical techniques.
Production of Therapeutic Metabolites
Metabolic fingerprinting of KC6 by GC-MS
The metabolic structure and composition of a probiotic isolate in the culture supernatant bids a window for explicating the inclusive nature of the metabolites. The metabolite profile produce information about the nutritional as well as therapeutic effects over the host health and also on the collaborative effects of various dietary gears on the host’s gut health [40]. Recently, Mass- spectrometry has turned out to be a powerful tool in the field of metabolomics studies due to its vast dynamic range along with its reproducible quantitative capabilities and ability of analyzing samples with significant molecular complexity. In our study, the aliquots of 24 h old bacterial cells were filtered to obtain the exometabolites followed by the solvent extraction by means of methanol: chloroform: water solvent system as this particular solvent system had been reported to be the most straight forward, effective and reproducible extraction system for the purpose of extracting metabolites [23]. The ultimate supernatant of the LAB isolate was further analyzed via GC-MS approach. In L. rhamnosus KC6, (2,3,4,5,6-pentamethyl acetophenone); phenol 2, 4bis (1,1dimethylethyl); dodecyl acrylate; ethyl iso-allocholate; 13docosenamide,(Z); 2-methylhexacosane; 1-hexacosene; lactic acid; tributyl acetyl citrate; pyrrolo [1,2-a] pyrazine1,4-dione,hexahydro-; 2-propenoic acid,3(4methoxyphenyl)2ethylhexyl ester; tetrapentacontane,1,54 dibromo; 12-octahydroindolo (2,3-A) quinolizine were the 13 metabolic compounds documented in total as described and depicted in Table 2 and Fig1(a-f) respectively.
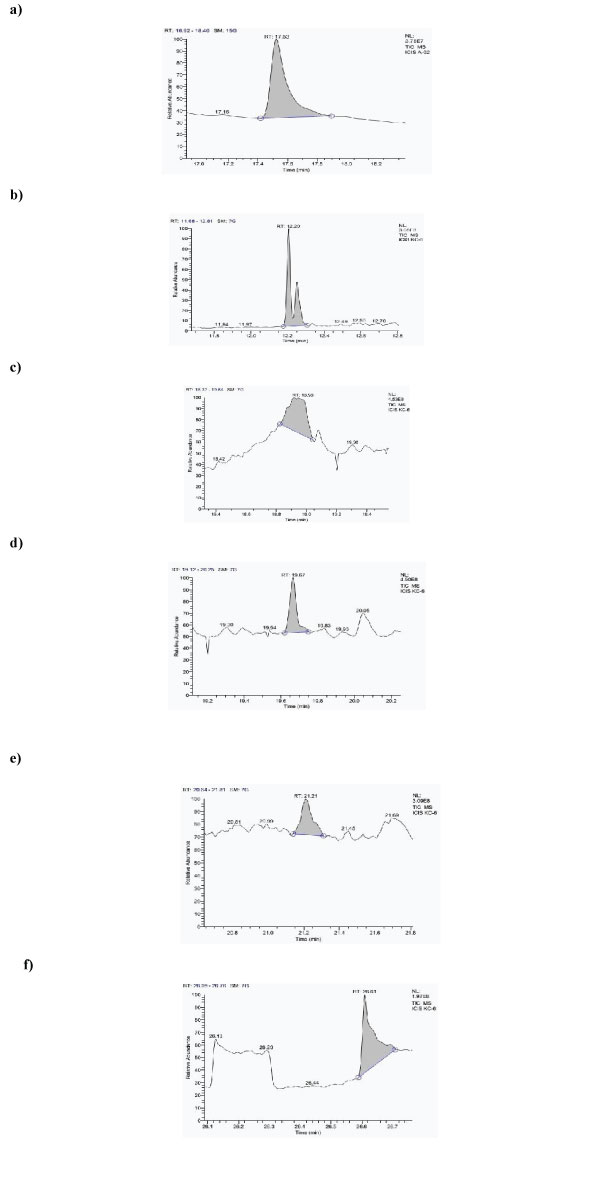
Figure 1: Chromatogram of metabolites found in L. rhamnosus KC6, a) 2-methylhexacosane, b) Dodecylacrylate, c) Propanoic acid, 3, 3'thiobisdidodecylester, d) 1-Hexacosene.e) Octadecane, 3-ethyl-5-(2-ethylbutyl) f) Tetrapentacontane,1,54-dibromo.
Table 2: Metabolic Compounds of KC6 obtained through GC-MS.
Sr. no. |
Metabolites |
Peak area shown by KC6 |
Therapeutic Effects of Metabolites |
References |
1 |
(2,3,4,5,6-Pentamethyl acetophenone) |
144190990 |
Used therapeutically to treat psoriasis and various types of neoplasms. |
[41] |
2 |
Phenol, 2, 4bis(1,1dimethylethyl) |
505242651.6 |
Down-regulation of the fimA, fimC, flhD and bsmA genes involved in biofilm formation. Used to enhance the efficacy of conventional antibiotics. |
[42] |
3 |
Dodecyl acrylate |
620049076.8 |
General adhesives and binding agents for a variety of uses like food and pharmaceutical industries. |
[43] |
4 |
Ethyl iso-allocholate |
1195845989 |
Cytotoxicity against A549 cells of lung cancer in vitro and in vivo. It reduces tumor growth, liver metastasis, and angiogenesis. |
[44] |
5 |
13 Docosenamide,(Z) |
553045462.5 |
A neuroactive compound in human and animals produced in response to glucose. |
[45] |
6 |
2-methylhexacosane |
390735693.1 |
Anti-inflammatory, antidiabetic and anticancer activity. |
[46] |
7 |
1-Hexacosene |
297265990.2 |
Potential analgesic effects which are most likely to be mediated by their anti-inflammatory activities. |
[47] |
8 |
796798270.1 |
A very essential compound for the chemical and food industry, used as a natural acidifying agent. Helpful in reducing skin wrinkles by topical application. |
[48] |
|
Metabolism of sugars to energy; Lactic acid was one of active ingredients in manufacturing of Phexxi, a non-hormonal contraceptive agent approved by the FDA on May 2020. |
[49] |
|||
9 |
Tributyl acetylcitrate |
61676554.51 |
Most frequently used plastifier in nail polishes, recommended to be used by people undergoing treatments that cause increased photosensitivity of the nails. Provide protection to the nails after the person has washed their hands. |
[50] |
10 |
Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro |
167746797.8 |
Antimicrobial and antioxidant activity against various infections. Potential anticancer agent. |
[51, 52] |
11 |
2Propenoic acid, 3(4methoxyphenyl)-2ethylhexylester |
229422327.7 |
Antibacterial and Antifungal activities |
[53] |
12 |
Tetrapentacontane, 1,54dibromo |
297265990.2 |
Antibacterial activities |
[53] |
13 |
12- Octahydroindolo (2,3-A) Quinolizine |
217293414.2 |
Anti-hypersensitivity activities |
[54] |
According to the literature, each of the above-mentioned bioactive compound is capable of conferring health benefits in one or another way. According to the recent updates of NCBI, (2,3,4,5,6-pentamethyl acetophenone) is used therapeutically in treating psoriasis and various types of neoplasms [41]. Ethyl iso-allocholate has also been suggested to display cytotoxicity against A549 cells of lung cancer in vitro and in vivo. It reduces tumor growth, liver metastasis, and angiogenesis [44]. 13 Docosenamide, (Z) specifically acts as a neuroactive compound in humans as well as animals and is produced in retaliation to glucose [45]. 2-methylhexacosane is known to exhibit antiinflammatory, antidiabetic and anticancer activity [46]. Lactic acid - (S)-2-hydroxypropanoic acid is considered to be an essential compound for the chemical and food industry, used as a natural acidifying agent. It is also helpful in reducing skin wrinkles by topical application. Moreover, it displays an efficient metabolism of sugars to energy and nonetheless, lactic acid was also one of the active ingredients used in the manufacturing of in Phexxi which is a non-hormonal contraceptive agent that had been approved by the FDA on May 2020 [48,49]. Tributyl acetylcitrate is a most frequently used plastifier in nail polishes and is highly recommended to be used by people undergoing treatments that cause increased photosensitivity of the nails as it provides protection to the nails of such people [50]. Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro antimicrobial and antioxidant activity against various infections. It is a potential anticancer agent as well [51,52]. 2-propenoic acid, 3(4methoxyphenyl)2ethylhexylester and tetrapentacontane, 1, 54-dibromo exhibit antibacterial and antifungal activities [53] and 12-octahydroindolo (2,3-A) quinolizine is known for its anti-hypersensitivity activities [54]. Moreover, many researchers have also observed that the metabolic capacities of lactic acid bacteria are responsible for the development or enhancement of the flavor, aroma as well as texture in different food products and also deliver therapeutic benefits by preventing or curing several diseases. In a study on lactic acid bacteria biota carried out by Reale and his coworkers, GC-MS analysis was performed in order to study the aromatic profiles of Italian traditional sourdoughs. Consequently, they recorded a variety of volatile compounds conjointly with the microbial metabolic profiles. Some of these metabolites play a vital role in health and well-being beyond the basic nutrition while some had also been reported as antimicrobial, antioxidant, anti-inflammatory, anti-cancer agent and anti-sleep disorder agents [55].
Antimicrobial Assay
Antagonistic activity of selected lactic acid bacterium was further tested against selected pathogenic bacteria viz. Bacillus cereus CRI, Clostridium perfringenes MTCC 1739, Escherichia coli IGMC and Staphylococcus aureus IGMC. Assessment of probiotic antimicrobial activity is considered a significant criterion for the inhibition of pathogenic intestinal microflora hence, protecting the host against their destructive action. The inhibition of the growth of pathogens can be through the production of antimicrobial compounds like organic acid, hydrogen peroxide, bacteriocins, etc. Lactobacilli are naturally present in human GI tract in addition to other natural microflora. These fermentative microorganisms produce certain organic acids including acetic and lactic acids, which significantly decrease the host’s intestinal pH and thus, inhibit the growth of undesirable microbial population in such microecological environment [56]. In general, they are extremely specific in terms of their activity and can kill the target bacteria by acting at the cell surface levels (i.e., on the membrane or cell-wall) and thus are potentially useful for human gut as well [57]. So, in the present study also, we assessed the antimicrobial potential of KC6 and the inhibition zones were measured on the test indicators lawns viz; 22mm for B. cereus, 27mm for C. perfringens 26mm for E. coli, and 25mm for S. aureus, during the growth cycle of KC6 after 44h of incubation as depicted in Fig2(a-d). Based on these results, it was concluded that the lactic acid bacterium showed wider zones in the supernatant depicting higher antimicrobial activity, thus, the isolate could possibly be an interesting asset for the purpose of helping the new biomedical therapeutics development. Dean-Scott and his coworkers investigated the natural antimicrobial potential of bacteriocinogenic Lactobacillus acidophilus and the study showed positive results for the same and it was then concluded that the isolate could be further useful in pharmaceutical or therapeutic applications [58].
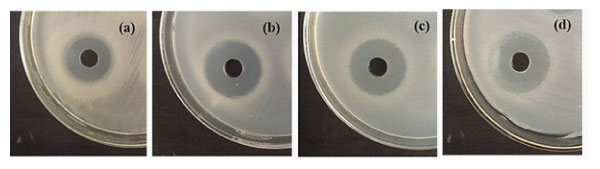
Figure 2: Antagonistic activity of L. rhamnosus KC6 against, a) B. cereus, b) C. perfringens, c) E. coli and d) S. aureus.
Therapeutic Effects
Determination of Antioxidant Activity
Probiotic micro-organisms generally express antioxidant potential by secreting certain enzymes like super-oxide dismutase (SOD) and promote the production of major non-enzymatic antioxidant molecules as well as glutathione (GSH)-a free radical scavenger which are quite helpful in maintaining oxidative metabolism of gut [59]. In our study also, the antioxidant potential of L. rhamnosus KC6 was evaluated via determining the DPPH scavenging capability in their cell free extracts. As an output, L. rhamnosus KC6 has shown 87.17% antioxidant activity Fig3. This remarkable antioxidative strength of the isolate assured that it can be useful in the improvement of the nutritional along with the functional properties of food and can thus help in combatting the oxidative stress in humans. In a study, Adebayo-Tayo et al. carried out an in vitro assay for evaluating antioxidant activity of Lactobacillus delbureckii subsp. bulgaricus and as a result of this, the overall DPPH scavenging activity came out to be ranging from 38.5-73.4% and 37.5–65.6%, while the total antioxidant capacity came out to be between the range 1.21% to 1.80% and 0.41% to 1.42 % in the strain [60].
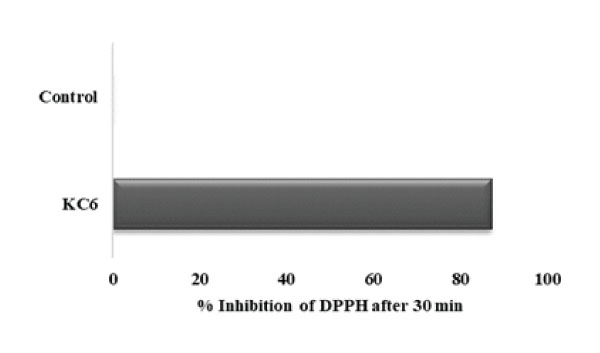
Figure 3: Antioxidant activity of KC6: L. rhamnosus KC6 has exhibited 87.17% antioxidant activity while the control devoid of the strain showed no activity.
Lipid Peroxidation Inhibition Activity of LAB in a Liposome System
Lipid peroxidation refers to a type of oxidative change in the polyunsaturated fatty acids located in the cell membranes and specifically result in the formation of numerous degradation LPO products like that of MDA which can potentially interrupt a chemical reaction by means of TBA. Usually, H2O2 system is used for catalyzing the oxidation reaction and the MDA level signifies degree of lipid-peroxidation in vitro. Thus, it is straightforwardly clear that anti-lipid-peroxidation activity is correlated with hydroxyl radical scavenging activity [61]. Here, in this study, L. rhamnosus KC6 has shown a percent inhibition of 60.33% in liposome system (Fig4). As in vitro, if the isolate has lipid peroxidation inhibition >50%, it possesses good antioxidant properties and is considered to be highly protective against any probable damage caused by the lipid peroxidation reactions, hence the isolate under the present study possesses the potential to protect the host property. In a study, Zhang et al. screened new LAB strains that were isolated from traditional Chinese fermented vegetable and alongside checked their resistance against the oxidative stress as well. As a result of this particular study, the Lactobacillus fermentum JX306 strain showed a strong in vitro lipid peroxidation inhibition rate which assured its possible use in therapeutic purposes in future [62].
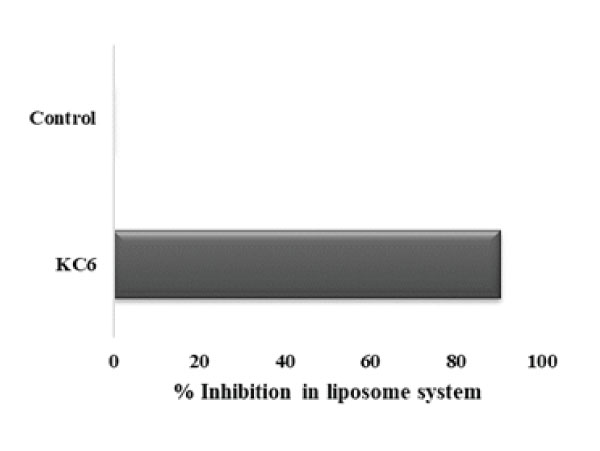
Figure 4: Lipid peroxidation activity of KC6: L. rhamnosus KC6 has exhibited 90.41% inhibition activity in liposome system while the control devoid of the strain showed no activity.
In Vitro Cholesterol Lowering Property
Cholesterol assimilation by the probiotics not only helps in the purpose of minimizing the available cholesterol level but also in the integration of conjugated linoleic acid as well as vaccenic acid within the cell-membrane which intend to modify its fluidity and hence its permeability, and contribute towards the lipid metabolism regulation as well by dropping down the concentration of total cholesterol level, LDL cholesterol and triglycerides [63]. In several other studies as well, it has been reported that a fair amount of Lactobacillus cultures is capable of exerting potential hypocholesterolemic activity [64]. Therefore, the study of potential probiotic strains which are isolated from fermented food matrices that could preferably decrease the serum cholesterol concentration can really be of high commercial importance. We also evaluated L. rhamnosus KC6 for its cholesterol assimilation ability (Fig 5) and as a result, KC6 was detected to harbor tremendous cholesterol lowering property of 77.72% assimilation rate. As the isolate showed the cholesterol lowering property >40% so, it can be recommended for the future commercial applications as probiotics after proper in vivo studies and would be very helpful especially in case of cardiovascular risks. In a study, Ma et al. also isolated 85 LAB strains, out of which they evaluated 3 for their cholesterol lowering property. As a result of the study, the cholesterol reducing property of Lactobacillus plantarum came out to be 28.8% [65].
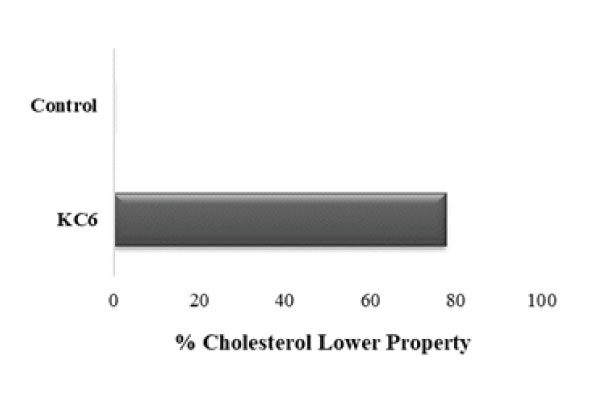
Figure 5: Cholesterol lowering property of KC6: L. rhamnosus KC6 showed 77.72% cholesterol lowering property.
Probiotic Properties
Tolerance Assays
Acid and Bile Salt Tolerance
There are several factors that affect the survival of probiotic bacteria within the gut, so, in case of probiotics, low pH tolerance of the probiotic strain is quite necessary factor for assessing their effectiveness. Alongside, the existence of bile salts and pancreatic enzymes form another hurdle for the probiotic survival during the process of digestion. Therefore, the capability of a probiotic strain to tolerate the intestinal bile salt is also of immense importance for their growth and survival in the GIT [66]. L. rhamnosus KC6 was found to be resistant to exposure to low pH for 3 h, proved by their remarkable growth at pH 3.0 when compared to that at physiologic pH, with minimal decrease in the growth rate that has been observed at pH 2.0. L. rhamnosus KC6 displayed highest % survival at pH 2.0 as well as pH 3.0 after an incubation time of 180 min as 97.68 and 95.97% respectively whereas the lowest was observed at pH 1.0 after an incubation period of 60 min as shown in Fig 6 (a). In case of bile salt tolerance, the results straightforwardly indicate that the strain is tolerant to physiologically pertinent concentration of 0.3% bile acid, with a drop-in cell viability observed at high bile salt concentration of 2%. L. rhamnosus KC6 tolerated maximum up to 95.75% after 4 h at 0.3% bile salt concentration and also expressed minimum survival of 80.74% after 8h at 2.0% bile salt concentration as shown in Fig 6 (b). It also showed maximum survival of 98.49% after 8 h at 0.3% bile salt concentration and minimum survival of 88.96% at 2.0% bile salt concentration after 8h of incubation. the lowest mean percent survival was reported in case of L. rhamnosus KC6 (94.76%) at the same concentration of 0.3%. So, it can be alleged that all KC6 could be a possible probiotic candidate with strong acid and bile salt withstanding properties, hence can be recommended as good probiotic strains for the future use as well. Evivie et al. in their study, testified the tolerance levels of Lactobacillus delbrueckii subsp. bulgaricus KLDS 1.0207 in acidic as well as bile salt conditions for the purpose of evaluating its efficacy as a potential probiotic. So, the subsequent results revealed that the strain could strongly withstand harsh stomach environment after 3 h of incubation in order to have specific functionality as well as beneficial effects which was a sign of its probiotic efficacy [67].
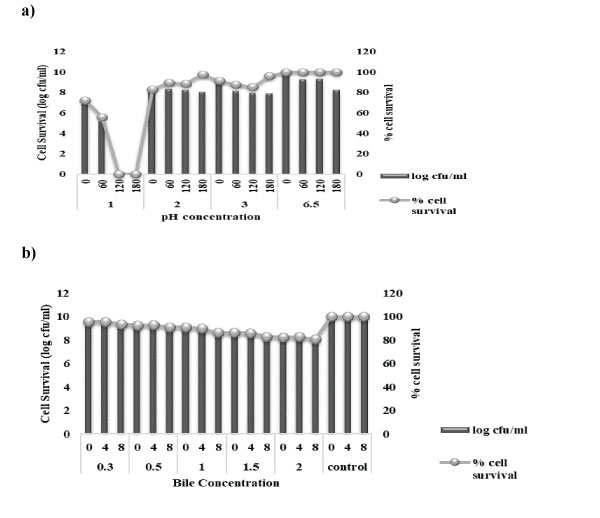
Figure 6: Acidity and Bile salt tolerance of KC6: a) KC6 is resistant to the exposure to low pH for 3 h, proved by their robust growth at pH 3.0 when compared to that at physiologic pH, with minimal decrease in the growth rate observed at pH 2.0. b) KC6 is tolerant to physiologically relevant concentration of 0.3% bile acid, with a drop-in cell viability observed at high bile salt concentration of 2%.
Tolerance to Simulated Gastric and Intestinal Juices
A robust and effective probiotic isolate is the one having the ability to survive as well as colonize under varied environmental conditions. Probiotics being usually orally administered, they must be able to survive throughout the gut passage across the stomach to the small intestine. Thus, the survival of the strain within simulated conditions mainly in the stomach and the duodenal passage was checked by incubating the selected LAB culture in MRS which was supplemented with pepsin (pH 2 and 3) and pancreatin (pH8) for a period of 4 h at 35°C. On the other hand, in order to assess the survival rate under simulated intestinal conditions, bacterial cells were cultivated in MRS broth supplemented with pancreatin (pH 8) mimicking the duodenal conditions and survival was observed till 4th h of incubation [29]. The isolate could sustain the simulated digestive conditions without any loss in the % cell survival. L. rhamnosus KC6 exhibited 73.23% and 76.80% of cell survival after treatment with simulated gastric juice (pepsin, pH 2 and pH 3) for 1h, respectively. L. rhamnosus KC6 was found to resist the gastric juice with percent cell survival of 64.88% and 73.08% after 2h of incubation at pH 2 and 3 respectively, as depicted in Fig 7. L. rhamnosus KC6 resisted gastric juice with percent cell survival of 32.11% and 55.31% after 4h of incubation at pH 2 and 3 respectively. The isolate under study successfully survived in both gastric as well as pancreatic digestion and this proves its capability in colonizing the gut.
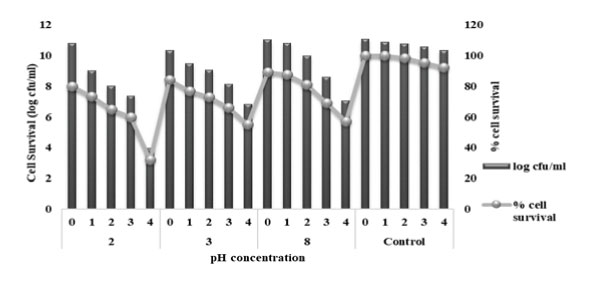
Figure 7: Tolerance of KC6 to simulated GI juices: L. rhamnosus KC6 resisted the intestinal juice with highest survival percentage of 64.88% after 4h of incubation under simulated intestinal conditions hence; it survived in both gastric and pancreatic digestion.
In general, majority of bacteria are unable to withstand the harsh environment conditions while travelling the upper GI transit involving both; the gastric as well as the duodenum. In comparison with many commercial strains, like L. casei LAFTI L26, Lactobacillus strain in the present study had been found to be highly stable under simulated GI environments. Results reported in several studies are also in consonance with the present results [68,69]. In a study, carried out by de Jesus et al. Lactobacillus delbrueckii subsp. lactis CIDCA 133 (CIDCA 133) had been testified as a potential probiotic strain. The CIDCA 133 was tested for the tolerance to the acidic gastric juice simulated with pepsin solution of pH 3.0 the results of which were in consonance with our present study [70].
Bacterial Auto-aggregation and Co-aggregation
Auto-aggregation is known to be the foremost step towards the adhesion process in the gut which preferably allows the bacteria to create a barrier for preventing the adhesion of pathogenic bacteria [71]. This proficient auto-aggregation permits to determine the actual ability of a bacterial strain to interact with itself in a nonspecific way. The bacterial aggregation which lies between cells of the identical strain (referred to as autoaggregation) or between different species or strains (referred to as co-aggregation) are the two most important characteristics to consider when evaluating a probiotic strain's ability to adhere in the oral cavity and the gastrointestinal tract as these are the areas where the probiotic strain is most likely to be active in conferring its benefits to the host. So, the auto-aggregation ability of the lactic acid bacterium was determined within a period of 5h of incubation. L. rhamnosus KC6 exhibited high autoaggregation percentage (89.8) as shown in Fig8(a) and on the other hand, in case of co-aggregation potential, L. rhamnosus KC6 was reported with 40.8% co-aggregation potential with B. cereus that was quite higher in comparison with C. perfringens and L. monocytogenes (i.e. 9.1% and 14.1%, respectively) as depicted in Fig8(b). As a probiotic bacterium which harbors good auto- as well as co-aggregation abilities are considered to have a remarkable potential of killing pathogenic bacteria via synthesizing certain antimicrobial substances; in this study as well, the selected LAB isolate has portrayed a good antimicrobial potential by displaying higher auto- and co-aggregation ability [72].
Sohn and his coworkers evaluated the probiotic attributes of Lactiplantibacillus plantarum LB5 which initially had been isolated from Korean radish-water kimchi (locally known as dongchimi). On the assessment of bacterial auto- and co-aggregation activity, LPLB5 exhibited a 30% and 33–60% auto-aggregation ability and co-aggregation ability with four pathogenic bacteria viz. E. coli O157: H7 ATCC 35150, E. coli KCTC 2571, L. monocytogenes ATCC 51776, and S. aureus ATCC 25923 respectively [73].
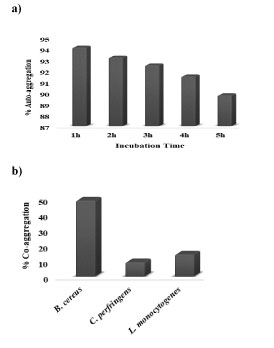
Figure 8: Auto-aggregation and Co-aggregation potential of KC6: a) auto-aggregation percentage of L. rhamnosus KC6 gradually decreased as a function of the incubation hour. b) highest co-aggregation was exhibited with B. cereus (40.8%) which was significantly high as compared to the co-aggregation ability of probiotic bacteria with L. monocytogenes (14.1%) and C. perfringens (9.1%).
Bacterial Adhesion Assays
Bacterial Adhesion to Hydrocarbons
The cell surface hydrophobicity (CSH) experiment helps in reviewing the adhesion capability of probiotic bacteria to epithelium in the GI tract, which leads to the prevention of pathogens colonization via the interaction between adhesive components and the complementary receptors [74]. The results in the present study for percent hydrophobicity of L. rhamnosus KC6 were marked as 89.0, 75.9 and 60.9% adhesion towards chloroform, xylene and ethyl acetate respectively. Hence, a good rate of hydrophobicity had been displayed by the isolate (Fig9). In a study, carried out over lactic acid bacteria which had been isolated from the Hu sheep milk, the hydrophobicity assay was demonstrated and the results elicited that among the four selected LAB isolates, the hydrophobicity of HSM-1, HSM10 and HSM-14 was higher in n-dodecane (i.e., 97.8, 97.9 and 97.2% respectively) as well as in xylene (i.e., 92.6, 94.6 and 93.4% respectively) which was much higher than that in chloroform (i.e., 82.5, 84.5 and 75.0% respectively), while the hydrophobicity of HSM-18 in chloroform (97.1%) was higher than that in n-dodecane (86.8 %) as well as in xylene (84.8 %). Therefore, HSM-10 was the one possessing the highest % hydrophobicity amongst all [75].
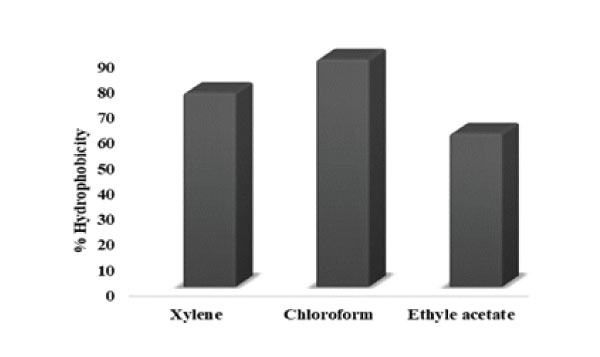
Figure 9: Adhesion of KC6 to hydrocarbons: 89.0% adhesion towards chloroform, 75.9% adhesion towards xylene and 60.9% towards ethyl acetate.
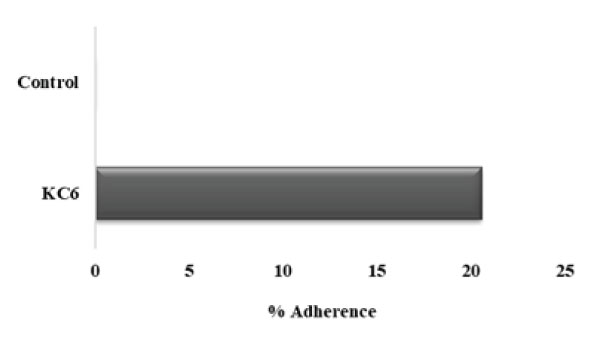
Figure 10: Adherence of KC6 to gastric mucin: L. rhamnosus KC6 20.56% adherence to the gastric mucin while the control devoid of the strain showed no activity.
Microbial Adhesion to Immobilized Gastric Mucin
When we talk about the selection of strong and good potential probiotic microorganisms having beneficial health effects on host organism, a number of criteria have to be accomplished for the fulfilling the ultimate purpose. Among them, one of the foremost criteria is the ability of adherence of the probiotics to the host tissue which is a very important asset for bacterial colonization. Next to the oral administration, probiotics travel through host’s GIT, where a wide range of physicochemical factors which are capable of affecting the survivability of probiotics [76]. Henceforth, the microbial adhesion as well as exclusion assays plays a very vital role in the study of any probiotic microorganism. In this study, the results demonstrated that the percent adherence of L. rhamnosus KC6 to be 20.56% (Fig10). In 2020, Mays attained similar results for the microbial adherence to the immobilized PIM (OD600 10.0) of L. reuteri ATCC PTA-6475 whereas the traditional assay displayed the equivalent as well as non-specific binding capacity of about 35% under all the applied conditions [77].
Displacement and Exclusion Assay
As the gastro-intestinal mucus is the most potent site for pathogenic attachment, hence, the colonization resistance along with competitive exclusion of pathogens is an essential property for the selection criteria of probiotic strains [78]. So, with this objective of evaluating the ability of probiotic lactic acid bacterium to prevent the pathogenic adhesion to gastric mucosa, these anti-adhesion assays had been carried out. Consequently, the pathogens exhibited strong adherence to immobilized gastric mucin (~108cells/mL) i.e. 164.8%, 483.2% and 458.4%, respectively. So firstly, in case of displacement of these pathogenic bacteria, L. rhamnosus KC6 exhibited 68.08% and 26.98% displacement for B. cereus and C. perfringens respectively. The probiotic bacterial strain was found able to displace B. cereus for the adhesion sites while it was unable to displace C. perfringens and L. monocytogenes (Fig11). In case of one-to-one competition between the probiotic bacterium and the pathogens, the competing ability of the probiotic isolate against these pathogens was examined. Conclusively, L. rhamnosus KC6 showed 30.96% degree of competition at 4h of incubation. The highest level of competition by probiotic bacteria was displayed against B. cereus as 64.86, 64.84, 67.05 and 66.10% followed by C. perfringens 50.87, 50.42, 52.25 and 54.68% and the lowest competition was showed against L. monocytogenes 5.08, 2.85 and 3.02% at 2h, 3h respectively at 4h of incubation but negligible in case of 1 hr incubation. (Fig12). This must be possible due to the different adherence sites between the pathogen and LAB at the mucosal receptors and as well they did not affect the adherence ability of each other [79]. It is clear that the selected strain can potentially prevent colonization of pathogenic bacteria and thus protects from risk of the spread of infections. Pinto et al. screened 280 lactic acid bacteria (LAB) which had been isolated from different food matrices and determined their probiotic characteristics including the survivability of the strains to the GI tract. Except for L. plantarum R23, all the isolates were capable of surviving through the simulated GI tract conditions but the overall result of the in vitro as well as in vivo study proposed that the strain P. pentosaceus CFF4 could potentially be used as a probiotic at commercial levels [80].
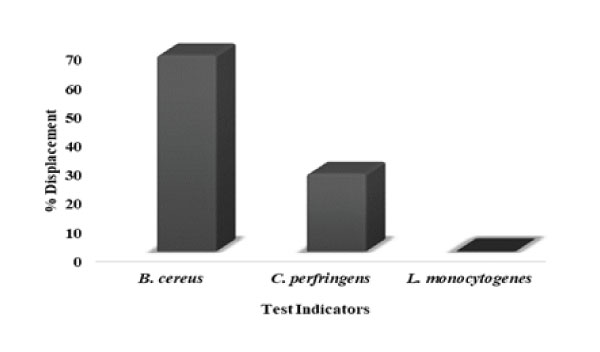
Figure 11: Displacement of pathogens shown by KC6: L. rhamnosus KC6 was found to be able to displace B. cereus for the adhesion sites while it was unable to displace C. perfringens and L. monocytogenes.
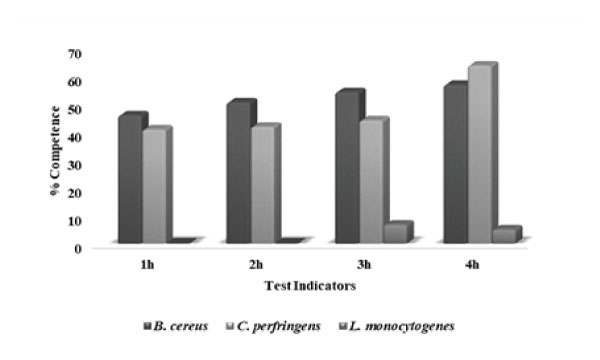
Figure 12: Competence against pathogens shown by KC6: L. rhamnosus KC6 exhibited highest degree of competition against B. cereus, followed by C. perfringens and L. monocytogenes upto 4h of incubation.
Safety Assessment
Enzyme Activity Assay
DNase and gelatinase enzyme are produced by most of the pathogenic bacteria. They interfere with the normal functioning of gut microbiome and cause certain infections, and those microorganisms which exhibit such property cannot be used as probiotics at all. So, we also determined the DNase activity of Lactobacillus isolate by witnessing the appearance of clear “halo” surrounding the isolate after flooding the agar with HCl solution while the production of gelatinase is signposted by the liquefaction of the medium and its tenacity after the refrigeration (hydrolysis of gelatin) [81]. In the present study, L. rhamnosus KC6 was found to be negative for the production of DNase (Fig13) as well as gelatinase (Fig14) enzyme and this ensures its safe status for the further therapeutic use. Abouloifa et al. evaluated the probiotic properties of 14 antifungal Lactobacillus strains which were isolated from traditional fermenting Moroccan green olives. On studying the DNase and gelatinase activity for safety assessment, the results revealed that none of the strains acquired any horizontally transferable DNase or gelatinase enzyme activities [82].
Hemolytic Activity
The reaction of hemolysis is referred to as a biological process which leads to the degradation of RBC membrane by a bacterial protein called hemolysin and as a result of this hemoglobin begins to leak out from the RBC, ultimately affecting the immune system of the person. During the in vitro assessment of this safety criterion, the absence of decolorized halo around the colony on blood agar plates indicates the negative hemolytic activity, i.e., no such degradation of RBC membrane has taken place by hemolysin [80]. In the present study also, negative results were observed for KC6 as can be seen in Fig15, therefore, proving its safe and non-virulent nature. Several reports have revealed that LAB do not show hemolysis [83,84] and our results is in consonance with them. Ebrahimi et al. in their study also assessed the LAB strain for hemolysis and as a result of this, in vitro safety of the Lacobacillus. kunkeei ENH01 was confirmed by negative blood hemolytic activity [84].
Antibiotic Susceptibility
According to WHO, 2002 and European Safety Authority-EFSA, 2008, bacteria which are used as probiotics in humans as well as animals must not carry any kind of transferable antimicrobial or antibiotic resistance gene [85]. Larger the number of transferable resistance genes present within the intestinal microbiota, higher would be the risk of pathogens getting hold within the GI tract resulting to the subsequent antibiotic treatment failure. So, antibiotic resistance is considered to be as a major aspect of safety assessment in order to evaluate the potential probiotics strains [86]. Our results in the present study disclosed that L. rhamnosus KC6 showed 88.8% of sensitivity towards the antibiotics, being resistant for amikacin (AK), amoxycillin (AMX), azythromycin (AZM), bacitracin (B), efpodoxime (CPD), cephalothin (CEP), chloramphenicol (C), ciprofloxacin (CIP), clindamycin (CD), erythromycin (E), gentamicin (GEN), norfloxacin (NX), novobiocin (NV), ofloxacin (OF), oxytetracycline (O) and penicillin-G (P) while susceptible for polymyxin B (PB) and vancomycin (VA) (Table 3 and Fig16) . Yerlikaya et al. also determined the antimicrobial activities and susceptibilities of nine Lactobacillus delbrueckii subsp. bulgaricus strains using the antibiotic discs, including ampicillin (10µg), ampicillin (25µg), bacitracin (10µg), clindamycin (2µg), clindamycin (10µg), erythromycin (10µg), erythromycin (15µg), gentamicin (10µg), gentamicin (120µg), nalidixic acid (30µ), neomycin (10µg), novobiocin (5µg), oxacillin (1µg), penicillin (10units), streptomycin (25µg), streptomycin (300µg), tetracycline (30µg) and vancomycin (30µg). The results showed that the strains LY6, LY8, LY9 and LY10 displayed remarkable antimicrobial activity for all the test microorganisms while each Lactobacillus bulgaricus strain came with resistance towards oxacillin (1μg) and nalidixic acid (30μg) and the highest antibiotic susceptibility was observed with that of ampicillin (25μg), clindamycin (10μg) as well as erythromycin (15μg) [87].
Table 3: Antibiogram of L. rhamnosus KC6.
Antibiotic |
Concentration |
Observed Results |
Amikacin (AK) |
10mcg |
S |
Amoxycillin (AMX) |
10mcg |
S |
Azythromycin (AZM) |
15mcg |
S |
Bacitracin (B) |
10units |
S |
Cefpodoxime (CPD) |
10mcg |
S |
Cephalothin (CEP) |
30mcg |
S |
Chloramphenicol (C) |
30mcg |
S |
Ciprofloxacin (CIP) |
5mcg |
S |
Clindamycin (CD) |
2mcg |
S |
Erythromycin (E) |
5mcg |
S |
Gentamicin (GEN) |
10mcg |
S |
Norfloxacin (NX) |
10mcg |
S |
Novobiocin (NV) |
5mcg |
S |
Ofloxacin (OF) |
5mcg |
S |
Oxytetracycline (O) |
30mcg |
S |
Penicillin-G (P) |
2units |
S |
Polymyxin B (PB) |
100units |
R |
Vancomycin (VA) |
10mcg |
R |
%Sensitivity |
88.8 |
Cumulative Probiotic Potential of KC6
The probiotic potential of the isolated strains is strictly based upon the assigned overall cumulative probiotic score. The cumulative probiotic potential is the sum total of the score of acid tolerance, bile salt tolerance, auto-aggregation, hydrophobicity, antibiotic susceptibility, etc. In the present study, probiotic potential for Lacticaseibacillus rhamnosus KC6 was adjudged quite high i.e. 82.5%. Usually, commercially available probiotics have probiotic score in the range or >80%]. Since the screened lactic acid bacterium in our study has qualified the prerequisite score, thus it can be recommended for the commercial use as a probiotic [88]. The present in vitro study discovered that these three potential isolates have fulfilled the criteria of FAO/ WHO in totality for meeting out the status of ideal probiotics [1]. Therefore, the study ensures their use in the development of novel nutraceuticals as well as affirms their therapeutic use, ultimately providing health benefits to the public and thus fulfilling the chief objective of the study.
Metabolic profiles of the L. rhamnosus KC6 came out to be quite advantageous for predicting its therapeutic characteristics. All the 13 metabolites analyzed in this study have numerous applications in therapeutic aspects. Some are helpful in conferring antimicrobial activity, antimutagenic, anticarcinogenic activities while some in regulation of immune function, improving gastro-intestinal health and allergenic diseases such as food allergies, etc. They can be used in pharmaceutical preparation, medicines as well as food products and dietary supplements having potential health benefits. These attributes of probiotic LAB have been clarified by the production of numerous primary and secondary metabolites which either directly or indirectly but potentially exert certain biological effects via immunomodulatory activities. Hence, KC6 can be considered as tremendous potential strain conferring good heath to public as it is safe as well as highly efficient probiotic. Moreover, the strain came up with tremendous results in various experiments performed for evaluating its therapeutic attributes including high antioxidant and lipid peroxidation activities along with good cholesterol lowering property in vitro. The strain exhibited all the essential probiotic properties which would be helpful in reducing the negative impact of viral infections as well and could help public in combatting the ongoing pandemic of COVID-19 via enhancing the immune system, thereby. Even though, further in vivo evaluation is also required for confirming its further commercial use. So, it may be concluded that LAB strains isolated from nutritionally rich fermented food matrix can be potential probiotic strain with quite attractive attributes for their beneficial use in functional food products. Henceforth, the present study upholds the use of L. rhamnosus KC6 in development of new therapeutic compounds in the present pandemic situation as well as in future for assisting mankind with numerous nutritional, immunomodulatory as well as therapeutic beneficences. Consequently, given the confirmed therapeutic potentials of the probiotic strain, it can definitely be recommended for future use; since, COVID-19 is a new spreading viral infection with high mortality rates much more research is required to affirm the probiotics as a safe as well as effective therapeutic agent against COVID-19.
Acknowledgements
Authors gratefully acknowledge the financial support provided by the Department of Environmental Science and Technology, Govt. of H.P, India to carry out this piece of work.