Pendant drop tensiometry was usually used to determine the surface elasticity and different kinetic parameters from a modified dynamic surface tension measurement in order to characterize milk protein adsorption behavior to the air/water interface. During manufacturing of various dairy products, milk proteins undergo significant changes due to the application of technological and preservation processing including enzymatic hydrolysis, heat treatment and the change of the pH level. The aim of the present chapter was to investigate the relationship between parameters, which are derived from pendant drop tensiometry (dynamic surface tension and interfacial rheology via volume oscillation) and foaming properties (foam capacity and foam stability) of milk proteins (caseins and whey proteins) as a function of protein composition, heating processes and variation of pH level. As an example, the results of the interfacial properties of sodium caseinates revealed that the initial adsorption rate values of caseinates proteins were reduced and longer time to reach equilibrium conditions was needed when pH decreased and after heating treatment at 70°C and 90°C for 30 min. These findings confirmed the pronounced caseins precipitation at low pH and at high temperature values.
Keywords: Interfacial Tension Dynamics; Interfacial Rheology; Sodium Caseinates; Whey Proteins; Protein Adsorption; Heat Treatment.
Foams are commonly defined as colloidal dispersions of gas phase bubbles in a liquid phase. Overall, the volume of the dispersed phase is large as compared with that of the continuous phase. The difference in the density of both dispersed and continuous phases as well as the large interfacial area lead to a high interfacial tension (~72 mN/m) and hence, to an unstable system [1]. Proteins are generally used in to stabilize the foams system in food and other foam industries by reducing the interfacial tension at the air/water interface. For instance, foods aerated products including ice cream, whipped cream, meringue, nougat and chocolate mousses are very popular. Thus, milk proteins are commonly used for stabilization of various food products in dairy industry due to their functional properties [2]. They can be classified in two main groups according to their molecular structure with different rheological properties: the flexible caseins and globular whey proteins. Flexible caseins are characterized by having no tertiary structure including the mixtures of sodium caseinate, calcium caseinate, and acid casein [3]. Caseinates are well known by having exceptional emulsifying, foaming and gelling properties which have been thoroughly examined and studied by many authors [3,4,5,6]. In recent years, the use of caseinates as an ingredient in nutritional products, dietary preparations, and medical applications has increased; many of these preparations included foamed ones.
Milk protein molecules change their charging and surface activity with pH and temperature. Consequently, their foamability and interfacial properties can be altered. Several authors have examined the surface behavior of various milk proteins including caseinates and whey proteins with their focus on adsorption on interfaces as function of pH [3,6,7] and of heat treatment [8,9].
The dynamic surface tension parameters are the main determining factors which are directly associated with the foamability of proteins. For instance, a rapid decrease of the surface tension indicates a fast adsorption of proteins to the interface and hence a higher foamability and stabilization of the integrated air bubbles against coalescence [3]. Overall, interfacial properties are determined using various techniques, which can be divided into two main classes of techniques [10]. These techniques are dilatational rheology which can measure the response of the adsorbed protein to compressional deformation by changing the area while maintaining the same shape and shear rheological techniques which determines the response to shear deformation by varying the shape while the area remains constant [10,11]. Many techniques have been commonly used to measure the interfacial properties including, capillary rise, Wilhelmy plate, Du Noüy ring maximum bubble pressure, drop volume, sessile drop and pendant drop methods [10].
Among the techniques described above, the pendant drop technique is arguably the simplest, most robust and versatile method. It is considered suitable for dynamic IFT measurements of protein-based systems [12]. Zhou et al. [10] noted that Suitability for different system sis good contrary to the other techniques whose suitability is limited.
In different studies on foaming and emulsifying properties of cow proteins (caseins and whey proteins), surface tension have been analyzed using pendant-drop tensiometry which is considered as a very precise method [1,3,13-16]. However, comprehensive studies on the foaming and interfacial properties of camel milk proteins are missing. Therefore, the aim of the present chapter is to investigate the relationship between pendant-drop tensiometry parameters (surface tension and viscoelastic modulus) and foaming properties (Foam capacity and stability) of proteins in order to reveal their foaming behavior. Cow and camel milk proteins fractions including skimmed milk, sodium caseinates and whey proteins are used as proteins examples with relevance for industrial applications in foam manufacturing.
Measurement of Interfacial Tension
The formation of emulsions and foams is based on the same principle and is essentially carried out by the creation of an interface followed by the migration and spreading of the surfactants. Indeed, the contact between two immiscible phases (air/water and water/oil for example) is thermodynamically instable. Thus, the energy stored at the interface or the interfacial tension is very high and it leads to the destabilization of the system [17].
From a physical viewpoint, the interfacial tension is defined as the force acting parallel to the surface (liquid/air and two immiscible liquids) and at right angles to a line of unit length anywhere in the surface [18]. While from a molecular viewpoint, interfacial tension arise from the energy differences between molecules in the bulk phase with each other and molecules associating with an immiscible phase at the interface between thermodynamically incompatible phases [19].
Many techniques have been used to measure the interfacial tension of food proteins including Wilhelmy plate, drop volume, maximum bubble pressure, Du Noüy ring, sessile drop capillary rise and pendant drop tensiometry methods. However, these techniques have limitations for the analysis of protein-stabilized systems. For instance, the use of the Wilhelmy plate technique requires a zero value for the contact angle of the liquid at the plate, which is so difficult as the protein analyte readily adsorbs onto the plate and increases its hydrophobicity. Hence, this method is reported to be not appropriate for the measurement of dynamic adsorption of proteins since the time taken from interface formation to measurement is not considered [10,20]. For the Du Noüy ring, a thin wire which is submerged below the studied interface, moves through the studied interface to pull up the liquid meniscus. The moving of the ring leads to disturbance of the studied film leading to major experimental error [10]. Drop lume and maximum bubble pressure are other techniques for the measurement of the interfacial tension.
However, they are not recommended for the study of adsorption kinetics in proteins [10,12].
Pendant Drop Tensiometer and the Measured Parameters
The axisymmetric air/water drop is formed at the tip of the needle of a syringe whose plunger position was driven by a computer. The image of the drop was taken from a CDD camera and then digitized. The interfacial tension is calculated by analyzing the profile of the drop according to the Laplace equation. The Laplace equation (Eq. (1)) reflects the balance between interfacial tension and acceleration gravity. It relates the density of liquids across the interface, the curvature of the drop profile:
(1/x) d(x sin θ)/dx = (2/b) − cz,(1)
where x and z are the Cartesian coordinates at the drop profile, b is the radius of curvature of the drop apex, c is called the capillary constant (equal to g△ρ/γ, where △ρ is the difference between drop phase and the continuous phase, γ the surface tension and g the acceleration of gravity) and θ is the angle of the tangent to the drop profile [15,21,22]. The interfacial tension is determined as a function of time (Figure 1). Sometimes, it is converted into surface pressure or interfacial pressure for easy comparison for systems with different γ0. Which is defined as the difference between the surface tensions of the protein solution and the pure solvent [22].
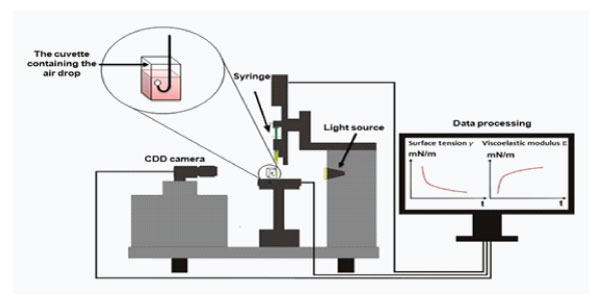
Figure 1: Pendant drop Tensiometer.
In addition to the interfacial tension parameter, pendant drop Tensiometer characterizes the interfacial dilatational rheology of the studied film protein at the surface of the created drop. Contact between two immiscible phases is thermodynamically instable, thus energy stored at the interface is very high leading to the destabilization of the system. Hence, the drop or bubble interface is subjected to sinusoidal compression and expansion through pendant drop Tensiometer by decreasing and increasing the drop volume at defined deformation amplitude (dA/A) and frequency. The drop shape is recorded in real time for subsequent fitting of Young
Laplace equation [10]. Overall, the dilatation frequencies are kept below 0.1 Hz in order to avoid false values of the viscoelastic modulus due to profile deviation from the Laplace shape [26].
Interfacial dilatational rheology relates variations in interfacial tension dγ, to infinitesimal changes in interfacial area, dA/A or dlnA. The viscoelastic modulus describes the elasticity of the thin liquid films, it is defined as the ratio of dγ and dA/A in Eq. (2) [15,22,25,27]. It is a measure of the dilatational viscoelasticity of the adsorbed film at an interface.
|ε|=dγdA/A=dγdlnA (2)
where A is the surface area of the drop. The control unit records and plots both the area fluctuation (dA) and the resulting sinusoidal interfacial tension fluctuations (dγ). The software can be used to plot the fluctuating interfacial tension against the relative variations in drop area [15].
The elastic component ε’ and the viscous component ε’’ are calculated from |ε| and the phase angle φ according to the following expressions:
|ε’|=|ε| cosφ,(3)
|ε’’|=|ε| sinφ(4)
From the interfacial tension curves, the adsorption kinetic parameters can be determined in order to characterize the rate of surface tension decrease when a new surface is created which is similar to the foaming process [28]. Hence, the adsorption rate (AR) of the protein at drop surface is defined as the initial slope value of the surface tension curve (AR = −dγ(t)/dt|t = 0) [3,22,24].
The measurement of the surface tension (γ) and viscoelastic moduli (ε) depends on the protein concentration. As expected, the interfacial tension decreased faster with increasing the protein concentration for both sodium caseinates and whey protein concentrate including 0.025%, 0.1% and 1% (w/w) as noted by Marinova et al. [3]. Meanwhile, Cases et al. [15] reported that when experiments were carried out at a concentration of 11 mg/L of proteins, the created interface would be fully covered by the tested proteins and only a very small amount of protein remained in the bulk phase, which is necessary for the clarity of the medium.
Characteristics of Milk Proteins
The functional properties of milk proteins are closely dependent on their physicochemical properties (molecular weight, nature and distribution of amino-acids, electronegative charge), their environment (pH, temperature, ionic strength, protein concentration) and the applied process (heating, drying, chemical or enzymatic modifications) [29].
According to their particular molecular structure, milk proteins can be classified in two groups: flexible and globular proteins [2,3,30]. Flexible caseins include the individual caseins αS1, αS2, β and k as well as caseins mixture such as sodium caseinates, calcium caseinates, acid caseins and rennet caseins. These proteins are distinguished by the absence of the tertiary structure leading to a higher flexibility. On the other hand,globular proteins, which can be isolated after casein precipitation upon cheese making process, are called whey proteins. These proteins are characterized by the presence of the buried disulfide bridges in their structure as well as the tertiary structure. The molecular characteristics of whey proteins lead them to be less tensioactive at the interface, furthermore, the preserve their globular molecular shape even after adsorption on an interface [3].
The molecular weight represents an important parameter controlling foaming and interfacial properties of proteins. Overall, proteins with a lower molecular weight are more competitive to the interface than proteins with a high molecular weight. For instance, serum albumin has lower techno-functional properties because of its high molecular weight (66 kDa) when compared to the α-lactalbumin (14 kDa) and the β -lactoglobulin (18 kDa) [29]. However, there are many contradictory reports on the interfacial properties of α-lactalbumin and β -lactoglobulin.
According to Closs et al. [31] and Slack et al. [32], the β -lactoglobulin has a better foaming, emulsifying and interfacial properties when compared to the α-lactalbumin despite its lower molecular weight. Indeed, the α-lactalbumin is a small protein that has good foaming properties but poor ability to stabilize the foam created. Thus, it migrates easily at the interface due to its low molecular weight (14 kDa) but it is unable to preserve the consistence of the created film [32]. On the contrary, Reimerdes and Lorenzen [33] and De Wit et al. [34] noted that the α-lactalbumin showed a 3 to 4 times greater emulsion stability than that obtained with β -lactoglobulin
The different molecular structure of the different proteins leads to different adsorption behavior with very different surface rheological properties [3,14,35,36,37]. Despite these known differences, a convincing explanation for the differences in the foaming and emulsifying properties of the two protein types is still missing in the literature. Caseins adsorption layers can be modeled by the β -casein alone as train–loop–tail model; indeed, the first 50 aminoacid residues of this protein are predominantly hydrophilic, while the remaining 159 residues are predominantly hydrophobic [3,35]. Neutron reflectivity studies of Dickinson et al. [36] showed that the adsorbed layer of β -casein molecules can be represented as an inner dense layer adjacent to the interface with a thickness of 1–2.5 nm composed mostly from the hydrophobic aminoresidues in a ‘‘train’’ configuration, meanwhile, the outer less dense layer, extending 3–7.5 nm further into the aqueous phase as a ‘‘tail’’ or ‘‘loop’’ representing the hydrophilic chain [36].
Modeling the adsorption layers of whey proteins is so different from that of caseins. Indeed, previous studies reported that modeling with single protein is controversial despite the dominance of the β -lactoglobulin because of the different surface properties of whey proteins and the β -lactoglobulin alone [3,22,38,39]. Zhang et al. [39] noted that the proportions of the main whey proteins including β -lactoglobulin to α-lactalbumin in whey foams depended on the pH level of the protein solution. Hence, the adsorbed layer from the whey protein mixture is composed of an average of globular proteins (Figure 2). This average of globular protein molecule adsorbs almost intact at the interface [40].
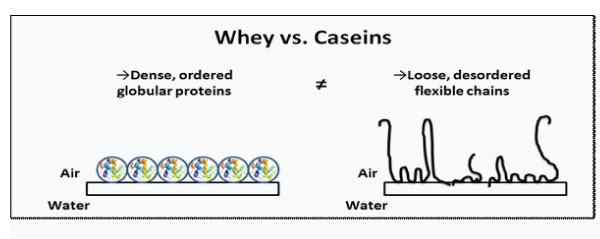
Figure 2: Schematic presentation of adsorbed whey proteins and caseins at air/water interface.
Characterization of the Foaming Properties and the Interfacial Behavior of Protein Solutions Using Pendant Drop Tensiometer.
Foaming and Interfacial Properties of Whey Proteins: Effect of pH and Heating Process:
The foaming properties of whey proteins were highest near the effective isoelectric point
(pI) which is around the pH level of 4.5. The ratio of the β -lactoglobulin and α-lactalbumin was higher above neutrality compared with that at the original pH of the protein solution (pH 6.6) indicating the preferential adsorption of the β -lactoglobulin over α-lactalbumin in the foam phase at neutral pH. On the contrary, the α-lactalbumin was more competitive in acidic conditions than the β -lactoglobulin due to the changed conformation and quaternary structure of whey protein upon pH variations. Indeed, proteins foam best at pH levels in which the molecules are more flexible and less compact [39,41]. Zhang et al. [39] reported that pH had significant effect on the competitive adsorption to the foam phase of individual whey milk proteins. Indeed, at pH level less than 5, the α-lactalbumin loses its bound calcium ions leading to its molten globular state and as a result, it becomes more tensioactive. Meanwhile, the β lactoglobulin is reported to be more rigid and thermo-dynamically stable at low pH. Hence, the β -lactoglobulin becomes less competitive to interfacial adsorption in acidic conditions compared to the α-lactalbumin. Furthermore, the β -lactoglobulin associates to form octamers at pH ranging between 3 and 5, it can even aggregate with the α-lactalbumin at pH < 4 which has an antifoam effect in acidic conditions [9,39,42,43,44,45]. Indeed, aggregation of whey proteins near their pI could not deplete the protein available for adsorption on the air/water interface but the formed aggregates exhibit antifoam effect [3]. The high foaming properties of whey proteins in acidic conditions (pH 4.5) was explained by Zhang el al. [39] as the combined effect of the molten globular state of α-lactalbumin and the reduced electrostatic repulsion of proteins at the pH close to their pI. The pI values of whey proteins were 4.1-4.8 for the α-lactalbumin and 5.2 for the β -lactoglobulin. These findings are in great consistence with those of Marinova et al. [3] who noted a good correlation between the highest foaming properties of whey proteins and rate of surface tension decrease dγ/dt|t=0. Ideally, the pH 5 is reported to be the closest pH of both α-lactalbumin and β -lactoglobulin leading to more reduced electrostatic repulsion between proteins and hence, better foam properties. However the overall foamability of whey proteins was lower at pH 5 compared to pH 4.5 due to the absence of the molten globular state of the αlactalbumin [46]. Marinova et al. [3] reported that the average adsorbed globular protein molecule to the air/water interface are negatively charged at the natural pH. Thus, the electrostatic repulsion forces prevent the formation of a very dense adsorption layer. On the contrary, these molecules are not charged near their pI leading to a highest adsorption level (Figure 3). Upon salt addition the adsorbed monolayer might increase slightly but not significantly since some of the charged amino-acids could be inaccessible for effective electrostatic screening [3].
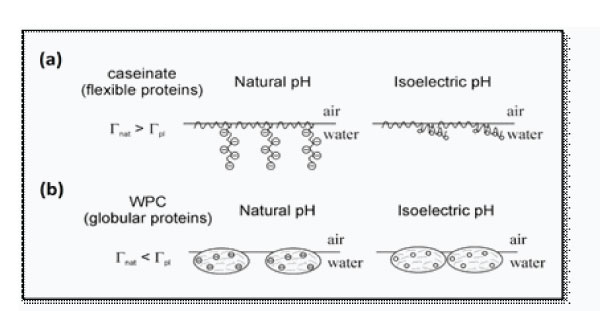
Figure 3: Schematic presentation of the effect of pH (7 and 4.5) on the adsorption of caseinates (a) and WPC (whey protein concentrate) (b) [3].
Pendant drop tensiometry analysis of Lajnaf et al. [22] evidenced that the order of effectiveness for bovine whey proteins was β -lactoglobulin (γ = 52.9 mN/m) > bovine whey (γ = 55.8 mN/m) > bovine α-lactalbumin (γ = 58.2 mN/m) for bovine proteins within 3000 s of the measurement, meanwhile, the deficiency of the β -lactoglobulin in camel whey leads to a similar surface tension kinetic evolution of camel whey and camel α-lactalbumin and a same final surface tension value at 3000 s (50 mN/m). Furthermore, the effectiveness of whey proteins in the creation of the most rigid surface film by bovine milk proteins were β lactoglobulin (ε = 50.3 mN/m) > bovine whey (ε = 45.6 mN/m) > bovine α-lactalbumin (ε = 37.3 mN/m), while for camel milk proteins, the order of efficiency was camel α-lactalbumin (ε = 23.2 mN/m) = camel whey (ε = 20.5 mN/m). On the other hand, these authors [22] reported that bovine whey showed an intermediate interfacial behavior between those of β -lactoglobulin and bovine α-lactalbumin alone, while camel whey and camel α-lactalbumin presented the similar foaming properties suggesting that foaming proteins of bovine whey are maintained by both α-lactalbumin and β -lactoglobulin, while these properties are mostly maintained by the αlactalbumin in camel milk due to its dominance. Pendant drop tensiometry was also used to evaluate the effect of pH and heating treatment on foaming and interfacial properties of whey proteins [13,23]. Lajnaf et al. [23] reported that that acid camel and bovine wheys presented higher foam capacity values when compared to their sweet counterparts, similarly they were more efficient in reducing the interfacial tension with final values of 51 mN/m after 3000 s. Interfacial and foaming properties of whey proteins depend also on their degree of denaturation after a heat treatment. Indeed, the applied thermal treatments at 70°C and 90°C for 30 min on camel and bovine whey proteins improved their foaming properties. Acid camel whey presented the best properties to create and to stabilize foams when compared to other whey, with an increase of these properties after a heat treatment. Similarly, the drop tensiometer analysis showed that acid camel whey preserved its tensioactive properties at the air/ water interface even after heating at 90°C [23].
Lajnaf et al. [47] noted that foaming and interfacial properties of the purified camel αlactalbumin were significantly improved by heat treatment at neutral pH. Furthermore, the stability of the foam made by camel α-lactalbumin greatly increased after heat treatment due to the presence of aggregates at the air/ water interface. Indeed, aggregates contributed to improve foam stability but slowed the adsorption of proteins and the creation of foam. The aggregated state, obtained after heat treatment and enhanced in acid conditions, is needed to greatly improve the foam stability of camel α-lactalbumin solutions [47].
Foaming and Interfacial Properties of Caseins: Effect of pH and Heating Process
Foaming and interfacial properties of sodium caseinates as a function of physicochemical conditions (pH, temperature and ionic strength) have been extensively studied by various authors [3,8,48–51]. Marinova et al. [3] noted that the surface tension value at the air/water interface decreased faster with increasing the protein concentration for sodium caseinates from 0.025% to 1% (w/w) (at all selected pH values and electrolyte concentrations). In the same way, foamscan experiments of Marinova et al. [3] carried out a solution of sodium caseinates at a concentration of 0.1 (w/w) leads to predict that bubbles with mean radius of 0.6 mm could be created from pores with mean radius of 15 mm if the surface tension value was 60 mN/m. Contrary to whey proteins, foamability of sodium caseinate is minimum near pI, meanwhile, at natural pH foaming properties of sodium caseinates was highest [3,39]. Indeed, the foaming of sodium caseinates solutions passed through a deep minimum at the isoelectric pI (4.5), upon decreasing the pH from the natural down to 3. These foaming properties changes were well correlated with the changes in the interfacial properties as well as a thin film lifetimes [3]. Marinova et al. [3] have modelled, through the pendant drop tensiometry assays, the adsorbed preacidified caseins at the air/water interface. They noted that the large distance between the hydrophilic residues of the adsorbed molecules would allow the electrolyte counter-ions to penetrate in between the different chains and thus to screen very effectively the electrostatic repulsion between the molecules in the adsorption layer (Figure 3). The depicted structure would allow such compaction to be realized even at minor screening electrolyte in the aqueous phase since the distance between the hydrophilic chains is large anyway due to the large area occupied by the hydrophobic segments at the surface [3].
Lajnaf et al. [22] found that skim milk, sodium caseinates and β -casein gave the highest foams and foams stability when compared to whey proteins such as β -lactoglobulin and αlactalbumin, reaching approximately FS~1000 s and FC~80.7% for extracted sodium caseinates. On the other hand, pendant drop tensiometry analysis of Lajnaf et al. [22] showed that that bovine sodium caseinates presented the best interfacial properties at the air/water interface when compared the pure bovine β -casein, bovine skimmed milk and whey proteins. It achieved a value of 47.1 mN/m within an hour starting from initial surface tension value around 72.8 mN/m with an adsorption rate value of f 0.252 mN/m/s. While camel sodium caseinates presented an intermediate interfacial behavior between that of skimmed milk and camel β casein reaching and surface tension value of 47.6 mN/m. These authors concluded that the β casein has the main role in the creation of camel milk foams at neutral pH as this protein was the most surface-active among all the studied milk proteins fractions (whey, camel αlactalbumin and caseins) [22]. On the other hand, camel and bovine sodium caseinates were reported to give the lowest viscoelastic modulus (ε~13 mN/m) at a protein concentration of 11 mg/L and hence, the least rigid adsorbed protein film at the air/water interface when compared to all the studied camel and bovine milk proteins fractions in this work (whey, α-lactalbumin, β -lactoglobulin, β -casein and skimmed milk) [22]. In the same way, Seta et al. [14] noted that proteins with a lower molecular weight and greater flexibility and that can adsorb and rearrange quickly at the interfaces, are usually expected to give lower dilatational viscoelastic modulus due to the more rapid recovery in the surface tension value at short and long times.
Hence, Lajnaf et al. [25] reported that the interfacial properties of milk proteins binary mixtures are mainly dominated by the presence β -casein: mixtures with higher β -casein contents are expected to be characterized by a more rapid recovery at the air/ water interface by giving lower surface tension and viscoelastic modulus values with higher foam capacity and foam stability values.
The pendant drop tensiometry analysis of Lajnaf et al. [22] reported that the dilatational rheological parameters have a close relationship with foaming properties of camel and bovine protein fractions. Globular whey proteins (whey, α-lactalbumin and β -lactoglobulin) exhibited the highest interfacial viscoelastic modulus values and the lowest ability to stabilize foams leading to suggest that the extent of protein rigidity made the molecular re-conformation more difficult but the resulting surface viscoelasticity was higher. On the contrary, milk and sodium caseinates and β -casein showed the lowest viscoelastic modulus values and the highest rate of adsorption leading to confirm that the extent of proteins flexibility is higher (especially for caseins), the molecular re-conformation at the air/water interface easier due to the weaker surface viscoelasticity [14,22,25].
Hence, Lajnaf et al. [22] have modelled the adsorption layers of bovine and camel proteins due to the pendant drop tensiometry analysis as follows: first, the β -casein polypeptide is adsorbed as inner adjacent layer at the air/water interface as train–loop–tail model [36] followed by the adsorption of β -lactoglobulin dimers and α-lactalbumin monomers with a preferential adsorption of the β -lactoglobulin dimers leading to an increase in the rigidity of created milk film. Meanwhile, the deficiency of the β -lactoglobulin in camel milk leads to a different modelling of the proteins adsorption layers as follows: α-lactalbumin monomers take the place of β -lactoglobulin dimers leading to a less rigid protein film compared to that of cow's milk [22].
The effect of heating process on foaming and interfacial properties of milk proteins were studied by various authors [48]. Lajnaf et al. [48] reported that foaming properties of sodium caseinates increased with increasing heating temperature process from 70°C to 100°C during 30 min due to the increase in the diffusion and adsorption velocity of these proteins at the interface and a decreased apparent viscosity upon heating. In the same way, these authors noted that heating treatment improved the efficiency of caseinates to reduce the surface tension values regardless of heating temperature using Du Nouy Ring tensiometry. On the other hand, pendant trop tensiometry results of sodium caseinates (Figure 4a and b) show that there is no observable significant effect of heat treatment at 70°C and 90°C during 30 min on bovine and camel caseinates. Even after heating caseinates, surface tension shape was found to depend mainly on pH value: a rapid decrease at pH 7 against a slower protein adsorption at pH 5 with a lower surface tension values at t=3000 s. After heating surface tension, results are similar when compared to the results at 20°C. Thus, interfacial properties of bovine and camel sodium caseinates are mainly governed by pH. Similarly, Mellema and Isenbart [13] have found that heating (85°C, 20 min) doesn’t affect the interfacial properties of skim milk proteins at oil water interface except gelling properties because of the heating effect on the microstructure and rheology of milk gels (Lajnaf et al. unpublished results).
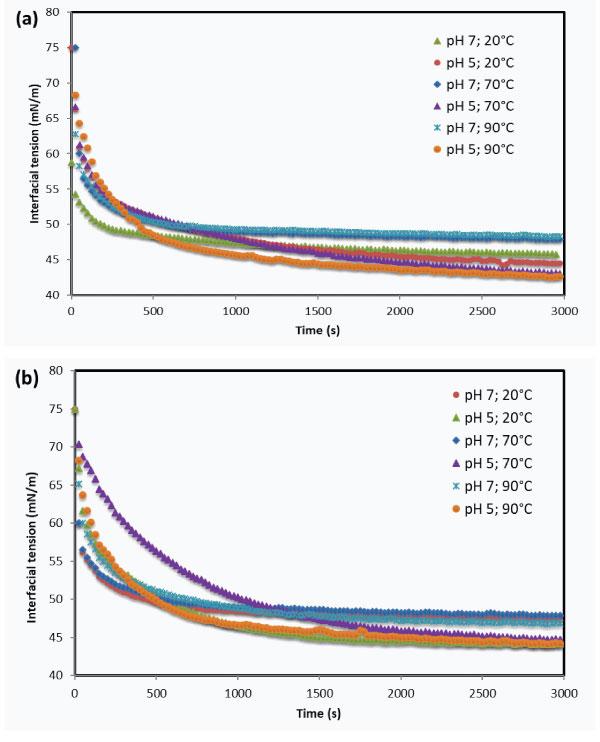
Figure 4: Pendant drop tensiometry results : time-dependent changes in interfacial tension γ (mN/m) at air/water interface for 10 mg/L of camel and bovine sodium caseinates solution as a function of heating temperature (20°C; 70°C and 90°C during 30min; bovine sodium caseinate (a), camel sodium caseinate (b).
Decreasing the pH of the bovine and camel caseinates from 7 to 5 induced a significant decrease of the initial adsorption rate from 0.67 to 0.35 mN/m/s respectively for camel caseinates and from 0.53 to 0.29 mN/m/s respectively for bovine caseinates. However, no significant effect of the heat treatment at 70°C and 90°C on the adsorption rate value was observed (Figure 5). These findings are in a great agreement with the results of Zhang et al. [39] and Marinova et al. [3] who reported that the foamability of milk decreased remarkably at pH values between pH 4 and 5 because of the precipitation of caseins. Thus, we would speculate that the decreased foamability which is due to the casein precipitation only and also to a lower surface coverage and a weaker repulsion between the surfaces of protein created film, which cannot ensure enough foam volume.
It has been shown in Figure 5 that camel caseinates exhibited the highest adsorption rate at pH 7 regardless of heating temperature however no significant difference has been observed between both bovine and camel caseinates at pH 5. This behavior may be due to the highest amount of the β -casein in camel caseinates and the lowest amount of the κ-casein. Thus, the β casein is the most flexible casein due to its unordered structure, whereas the κ-casein is the most structured casein which may contribute mainly to the foam stabilization. According to Meste et al. [52], the effectiveness of caseins in creating foams was β -casein > α-casein> κ-casein.
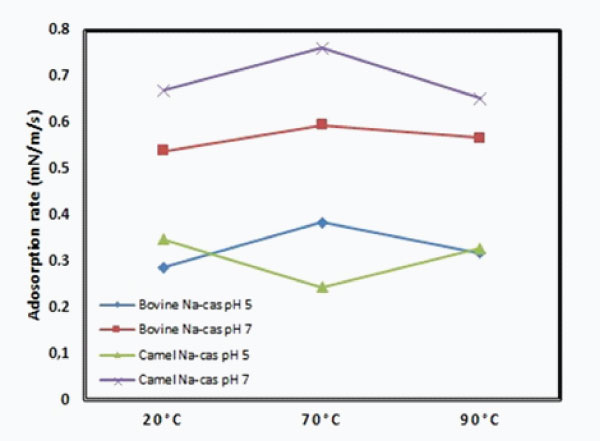
Figure 5: The adsorption rate of the 10-3 wt% bovine camel Nacas (rate of initial decrease of the dynamic surface tension, i.e. -dγ/ dt|t = 0). Abreviations: Na-cas: sodium caseinates.
The slower adsorption of caseins at pH 5 (Figure 4) is also caused by protein aggregation near their pI. Indeed, the aggregation of sodium caseinates reduces the number of individual proteins which are able to fix at the air/water interface decreasing the interfacial tension. Dan et al. [53] reported that β -casein has no net charge in acidic conditions.
The intermolecular interaction is favorable as compared to pH 7 where electrostatic repulsion took place. The aggregation is pronounced at pH 5, almost proteins interact and a lower β -casein concentration remained at the bulk of protein solution. A lower bulk concentration results in a slower adsorption of β -casein. Thus, no equilibrium has been reached and the dynamic surface tension continues to decrease over a longer period of time than pH 7 where the equilibrium is rapidly reached. Furthermore, at pH 7, the β -casein molecules are stretched due to a higher molecular charge and consequently, they penetrate more into the whole interface in tails and loops [53,54]. So the behavior of native camel and bovine caseinates at pH 5 and 7 can be explained which is in great agreement with previous foaming properties: caseins aggregates at low pH values leading to a lower created foam volume as aggregates adsorbed slower than a native form but once adsorbed, they give a more stable film than at neutral pH despite the higher electrostatic repulsion at this pH value.
Aim of the present chapter was to investigate the relationship between foaming properties of milk proteins and the different parameters which are derived from pendant drop tensiometry and foaming properties. Thus, pendant drop tensiometry was used to determine the surface elasticity as well as different parameters from a dynamic surface tension measurement to characterize milk protein adsorption (caseinates and whey proteins) to the air/ water interface as a function of denaturing conditions. Overall, previous results showed that for milk proteins in native conditions (neutral pH and room temperature), a significant relationship between pendant drop tensiometry parameters and foaming properties of both camel and bovine protein fractions.
For both milk samples: globular whey proteins (whey, α-lactalbumin and β -lactoglobulin) exhibited the highest interfacial viscoelastic modulus values and the lowest ability to stabilize foams, while caseins (β -casein and sodium caseinates), exhibited the lowest surface tension values and the highest ability to create and stabilize foams.
On the other hand, the denaturing conditions (heat treatment, and pH) of milk proteins had a significant impact on their foaming and interfacial properties. Hence, for the interpretation of data, the adsorption rate which represents values of the initial slopes of the interfacial tension curves a well as physicochemical parameters (ζ-potential, surface hydrophobicity and solubility) should be taken into consideration. Therefore, pendant drop tensiometry proved to be a valuable tool for the characterization and prediction of the foaming properties and foam stability of protein solutions especially under native conditions.