It remains common practice to chop the food of zoo-housed animals, even when the animals themselves are capable of processing larger food items. Zoo husbandry practices should be based on evidence, so there is a need to identify whether chopped food diets have any benefits for animals as opposed to whole items. Research was undertaken to investigate the effect of food condition (chopped or whole) for a bachelor group of ring-tailed lemurs Lemur catta. Lemur diets were prepared in both a chopped and whole format, with equal amounts of each ingredient being provided for each format. Both the chopped and the whole food were provided to the lemur group simultaneously, and the food intake, food preference, and behaviour of the lemurs was measured using instantaneous focal sampling. While lemurs ate slightly more whole food (161 grams) than chopped food (137 grams) per observation, this was not significant. Lemurs also tended to select whole food items more frequently, though this was also not significant. However, lemurs spent significantly longer feeding, and engaged more often in carrying behaviour, when selecting whole food items. No issues associated with aggression or stealing were observed. Overall, whole food items had beneficial effects in terms of lemur movement and feeding time. It is likely, therefore, that diets containing whole foods may have value in terms of lemur husbandry and management, encouraging more natural feeding strategies. This would have value in terms of saved keeper time, reduced nutrient breakdown of food, and reduced risks of contamination. Further studies on lemur food presentation could incorporate studies of food preference and food manipulation.
Keywords: Chopped Food; Food Presentation; Lemur Catta; Primate; Zoo
Food presentation in the zoo
Food provided for zoo animals is often chopped into small, bitesize pieces, even when the animals are capable of tackling much larger items (Plowman et al. 2006). The practice of chopping food is surprisingly common, with chopped foods used in the diets of myriad species for zoos and aquaria (Young 1997; Brereton 2020; Waasdorp et al. 2021). Chopping food possesses several disadvantages, including loss of keeper time (James et al. 2021), nutrient breakdown (Hodges and Toivonen 2008) and risk of bacterial contamination of food. From an evidence-based perspective, it is important to determine whether chopped food provides any benefits to the animals being fed (Melfi 2009).
The literature surrounding chopped food for zoo and aquarium animals is complex (Brereton 2020). Orange-winged amazons Amazona amazonica, when provided with regular or oversized pellets (of identical nutritional quality) showed a significant preference for the larger pellets (Rozek et al. 2010; Rozek and Millam 2011). The birds were also motivated to engage in contrafreeloading to gain access to the larger pellet type (Rozek et al. 2010; Rozek and Millam 2011). Blue and gold macaws Ara ararauna spent significantly more time engaged in podomanipulation and allofeeding when provided with whole fruit items as opposed to smaller pieces. For the coati, provision of whole foods reduced aggression (Shora et al. 2018).
Primates have been well studied in terms of food presentation research. Four papers focusing on macaques Macaca spp. have been undertaken (Smith et al. 1989; Mathy and Isbell 2001; Plowman et al. 2006; Sandri et al. 2017). The provision of whole foods increased the dietary diversity for one group of lion-tailed macaques Macaca silenus (Smith et al. 1989), likely because individual animals were not able to monopolise all resources. Similar findings were identified for the Sulawesi crested macaque Macaca nigra; while whole food provision did not affect food intake for most animals, the most subordinate individual was able to take in significantly more food than during chopped food diet provision. Barbary macaques Macaca sylvanus demonstrated a decrease in aggressive behaviour and an increase in affiliative behaviour when provided with whole food. An increase in aggression was noted only in the rhesus macaque Macaca mulatta (Mathy and Isbell 2001). However, only two food items were fed at a time to a large group of macaques, which is likely to have increased competition.
While macaques have been well studied, there remains scope to extend research on food presentation for a wider range of primate species. It is particularly important to engage in further research, because different primate species are likely to respond differently on account of their natural feeding ecology and social structure (Waasdorp et al. 2021). Prosimians, such as lemurs, represent an excellent opportunity to investigate food presentation impacts further (Britt 1998).
Lemur diets
The ring-tailed lemur Lemur catta, a diurnal, omnivorous Malagasy lemur, was recently reclassified as Endangered by the International Union for Conservation of Nature (IUCN) (Lafleur & Gould 2020). This species is well represented in zoos globally, with a reported population of over 4,500 individuals. Zoo housing strategies for ring-tailed lemurs vary between zoological collections, with some lemurs maintained in mixed-species enclosures, others in walk-through exhibits in large troops, and others maintaining pairs or bachelor groups.
In the wild, ring-tailed lemurs form mixed-sex troops, and forage for food on both the forest floor and in trees (Simmen et al. 2003; Mertl-Milhollen et al. 2013; Bennett et al. 2016). Females tend to be dominant to males, and aggressive interactions may take place over food resources (Junge et al. 2009). In their natural habitat, seasonal fluctuations in rainfall and weather conditions often result in periods of food shortage, during which lemurs adjust their diets in order to continue feeding (Bennett et al. 2016). Ring-tailed lemurs have been observed feeding on a range of fruits, leaves and buds, with occasional invertebrates selected (Dierenfeld and McCann 1999). In Madagascar, one of the most common food items is the leaves and fruit of the tamarind Tamarindus spp. (Mertl-Milhollen et al. 2013). Feeding on the fruits of this tree is believed to be associated with tooth wear and loss in older male lemurs (Bennett et al. 2016).
The digestive system of the ring-tailed lemur is equipped to cope with an omnivorous diet. This species possesses a haustrated hind gut, allowing some fermentation of fibre (Clauss et al., 2021). However, in captivity lemur diets are not always reflective of the wild, fibre-rich diet, and fruit may sometimes be provided in high quantities (Britt et al. 2015). Fruit-based diets have been linked with aggression across a range of lemur-species, and food presentation may potentially exacerbate these issues (Plowman et al. 2006; Britt et al. 2015).
Given the amount of time required to prepare diets for zoo animals, along with the detrimental effects on food quality and potential risk of microbiological contamination, there is a need to determine whether chopped or whole food diets have any impact on the behaviour and interactions between lemurs. It is also important to determine which food presentation techniques the lemurs themselves would prefer. As a result, this study was initiated, investigating the food preference and behaviour of a bachel group of ring-tailed lemurs when offered chopped or whole food diets.
Study subjects and location
The research presented in this study was ethically approved by Sparsholt’s Ethics and Research Standards Group prior to investigation (USCEC6721). The study took place from November 2021 to January 2022 and investigated the feeding behaviours and food preference of three male ring-tailed lemurs housed at Sparsholt College’s Animal Health & Welfare Research Centre (AH&WRC) (Table 1).
Table 1: Study subjects.
House name |
GAN |
Birth date |
Birth location |
Andy |
CFF18-23335 |
1 May 2018 |
Chester Zoo |
Llywelyn |
CFF19-24368 |
18 Apr 2019 |
Chester Zoo |
Morris |
CFF21-25775 |
3 Mar 2021 |
Chester Zoo |
The lemurs were housed in an indoor enclosure containing a climbing frame, and access to a larger outdoor exhibit (Figure 1). The indoor and outdoor exhibits were connected together by a wire mesh chute. Enrichment types varied regularly and included bamboo branches, branches, and leaf litter boxes. The lemurs were housed between two other lemur enclosures: a sole ring-tailed lemur and a group of 4 black lemurs (Eulemur macaco) but had no access to them even when outdoors.
Diet preparation
Lemurs were fed twice per day; morning feeds took place at 08:30 and evening feeds at 14:30. Both meals were observed during each observation day. Each observation period was two hours long totalling at 4 hours of observation per day. Food type varied depending on the day of the week, as per the normal husbandry for the lemurs (Table 2). During observations, half of the diet was chopped into small pieces (2x2cm3), and the other half was prepared in large chunks (6-10cm3, or slices, depending on food type) (Figure 2). The food was placed in the exhibit in large blue bowls, as per normal husbandry routine. One bowl contained the small food items, and the other contained the large items. The exact position of each bowl in the enclosure was randomised to prevent control for convenience of location effects. Both food bowls were weighed at the beginning of each observation, and again at the end of the two hour observation period. The amount of food eaten was calculated by deducting the remaining food and bowl weight from the starting weight.
Table 2: Diet of lemurs as per normal husbandry.
Day |
Time |
Food item and quantity (g) |
Monday |
08:30 |
Onion (70), celery (70), cucumber (120), bok choy (180), leaf eater pellet (140) |
14:30 |
Swede (180), squash (180), red pepper (150), parsnip (150) |
|
Tuesday |
08:30 |
Leek (90), courgette (120), fennel (60), chicory (180), leaf eater pellet (140) |
14:30 |
Carrot (180), sweet potato (180), beetroot (70), green breans (40), sweetcorn (180) |
|
Wednesday |
08:30 |
Onion (70), celery (70), cucumber (120), bok choy (180), leaf eater pellet (140) |
14:30 |
Swede (180), squash (180), red pepper (150), parsnip (150) |
|
Thursday |
08:30 |
Leek (90), courgette (120), fennel (60), chicory (180), leaf eater pellet (140) |
14:30 |
Carrot (200), sweet potato (180), beetroot (70), green breans (40), sweetcorn (180) |
|
Friday |
08:30 |
Onion (70), celery (70), cucumber (120), bok choy (180), leaf eater pellet (140) |
14:30 |
Swede (180), squash (180), red pepper (150), parsnip (150) |
|
Saturday |
08:30 |
Leek (90), courgette (120), fennel (60), chicory (180), leaf eater pellet (140) |
14:30 |
Carrot (180), sweet potato (180), beetroot (70), green breans (40), sweetcorn (180), broccoli (90) |
|
Sunday |
08:30 |
Onion (70), celery (70), cucumber (120), bok choy (180), leaf eater pellet (140) |
14:30 |
Swede (180), squash (180), red pepper (150), parsnip (150) |
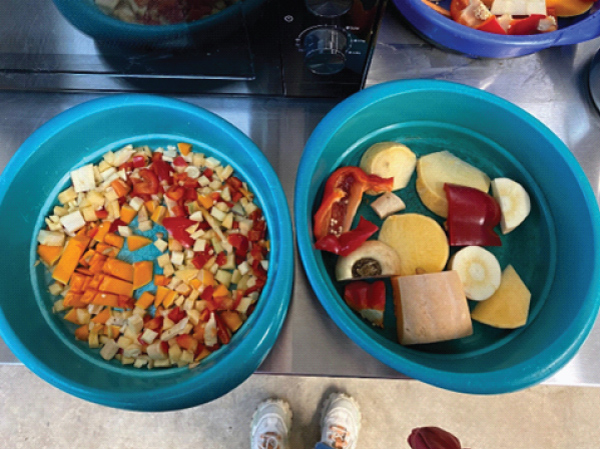
Figure 2: Example of diet prepared to ‘small’ and ‘large’ food particle sizes. The same food items and same quantities were provided for both conditions.
Behaviour sampling
The observation method chosen for this study was instantaneous focal sampling at 1-minute intervals. Behaviours were determined through an ethogram which was adapted from Shora et al. (2018) and Dierenfeld and McCann (1999) (Table 3). All lemurs could be individually identified. The observer sat quietly outside the exhibit but was within sight of the lemurs. The observer also recorded the first food item (small versus large) that each lemur selected during each observation, and the length of time that the lemurs took to select the first item per observation.
Table 3: State behaviours observed during study.
Behaviour |
Definition |
Allogrooming |
The lemur cleans another individual’s fur using its tongue and hands. |
Carrying food |
The lemur is moving with a piece of food, chewing food, or holding food away from the location of the food bowl. |
Chasing |
The act of one lemur moving around enclosure at a fast pace following another individual. |
Eating |
The act of chewing, manipulating or swallowing of food items. |
Grooming |
The lemur cleans its fur using its tongue and hands. |
Inactive |
The lemur is motionless and is sitting, resting or sleeping. |
Locomotion |
The act of, walking, jumping, running, climbing |
Playing |
The lemur interacts with another individual or with enrichment items in a non-aggressive manner. |
Data analysis
Data were collected into Microsoft™ Excel 2016, and uploaded to Minitab version 23 for analysis. First, data on food intake were tested for normality. As the data were not normally distributed, a Mann Whitney U test was used to determine whether there was a difference between amount eaten for the chopped and whole conditions.
For food preference, the first food item selected per lemur per observation was analysed using a binary logistic regression. Food type (chopped versus whole) was inputted as the response, and individual lemur and time of observation (morning versus afternoon) were inputted as predictors. For the amount of time taken to select the first food item, a regression was run, with the time taken to select the first food item (seconds) as the response, and the food condition (chopped or whole), time of day and individual lemur as predictors.
For behaviour analysis, Poisson regressions were run, with the behaviour as the response, and the time, individual lemur and food selection (small versus large) as the predictors. As the lemurs had access to both food conditions during all observations, food condition could not be assessed for all behaviours. However, for three behaviours (eating, chasing and carrying), the last food item eaten was recorded. Poisson regressions were therefore run only on these three behaviours.
Food intake
On average, lemurs tended to eat more of the whole food (161 grams) than the chopped food (137 grams) (Figure 3). However, this trend was not significant (W=841, n=30, P=0.277).
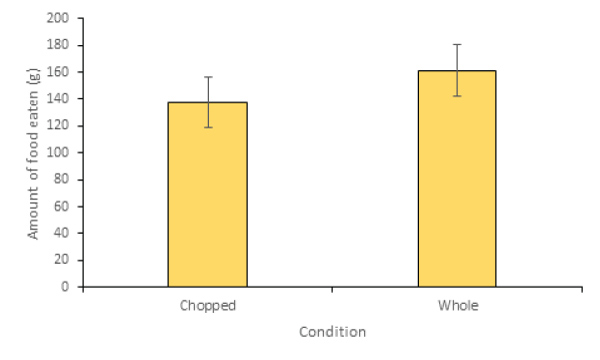
Figure 3: Amount of food eaten per observation for the chopped and whole food conditions (+/- standard error).
Food preference
Overall, all lemurs selected whole food more frequently as their first choice during observations (Figure 4). Neither the individual lemur nor the time was a significant predictor of food selection (Z=0.53, X2=0.05, P=0.997).
The time taken to select the first food item for both chopped and whole foods was calculated (Figure 5). The food type (chopped versus whole) was a significant predictor of time taken, with lemurs selecting whole food items significantly faster than chopped (X2=0.671, r2=1.23, SE=0.078, P=0.010). Individual lemur and time of day were not significant predictors and were excluded from the model.
Behaviour
Activity budgets were generated for all three lemurs (Figure 6). As lemurs had access to both chopped and whole food during all observations, and therefore could select both chopped and whole items within the same time block, the impact of food type could not be assessed for all behaviours. However, the food type selected was recorded whenever lemurs were observed carrying or eating food, and the last food item taken was recorded for chasing behaviour (Figure 7). Poisson regression models were run to identify the impact of food type, time of day and individual lemur on chasing, eating and carrying behaviour (Table 4). Food type and individual lemur were significant predictors of both eating and carrying, but time of day was a significant predictor only for carrying behaviour. There were no significant predictors of chasing.
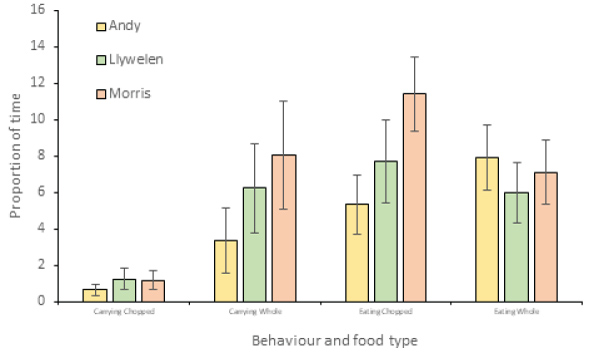
Figure 7: Proportion of time feeding and carrying large or small food items for the three study subjects.
Table 4: Outputs of Poisson regression models for eating and carrying behaviour.
Behaviour |
R2 (P) |
Predictor |
SE Predictor |
DF |
X2 |
P |
Carrying |
51.47% (<0.001) * |
Food type |
0.105 |
1 |
275.99 |
<0.001 * |
Individual |
0.106 |
2 |
69.29 |
<0.001 * |
||
Time |
0.082 |
1 |
107.09 |
<0.001 * |
||
Chasing |
4.36% (0.146) |
Food type |
0.678 |
1 |
0.01 |
0.921 |
Individual |
0.645 |
2 |
6.74 |
0.064 |
||
Time |
0.460 |
1 |
0.05 |
0.822 |
||
Eating |
7.83% (<0.001) * |
Food type |
0.051 |
1 |
9.49 |
0.002 * |
Individual |
0.066 |
2 |
40.67 |
<0.001 * |
||
Time |
0.051 |
1 |
0.43 |
0.511 |
While non-significant, black lemurs tended to select and ate slightly more of the whole food items during the study. The lemurs also spent significantly more time eating and carrying food when provided with whole items, though no impact on chasing was identified. This suggests that overall, whole food items may actually have benefits in terms of lemur activity budgets.
Food intake and preference
While lemurs tended to eat more of the whole food in comparison to chopped foods, the trend was not significant. Similar is true for the first choice of food item, with whole food items being selected slightly more often than chopped. Lemurs spent significantly less time selecting whole food items as their first choice, however, which may suggest greater feeding motivation when provided with these items.
Overall, information on food intake and preference is complex. While the results may indicate that whole food items are of interest to lemurs, both the chopped and whole wee eaten during observations. Ring-tailed lemurs tend to be selective in their feeding habits, taking carbohydrate-rich food items preferentially (Hansell et al. 2020). While the food items offered in both the chopped and whole conditions were identical, the actual food item taken by the lemurs (e.g. red pepper) was not recorded in the study, and this may act as a confounding factor. Additional food type effects could be incorporated into future studies.
It is important to note that lemurs did not appear to avoid the whole food items, and while a clear preference for whole foods was not identified, there were no concerns of neophobia identified. The lemurs also appeared to have no problems with processing the whole food items, as shown by the food intake data. This is in itself unsurprising, given that the wild ring-tailed lemur can successfully tackle tough-skinned fruits such as those of the tamarind tree (Mertl-Millhollen et al. 2013). However, this is promising, as the lemurs were not regularly exposed to whole food items prior to the start of the study.
Behaviour
Ring-tailed lemurs spent significantly longer periods of time engaged in feeding behaviour when they selected whole food items. The lemurs spent significantly longer carrying these food items, normally up to higher platforms within their enclosures in order to eat. Carrying of whole food items may be more effective, because lemurs could then spend longer periods of time feeding on the comparatively larger items. This potentially reduced competition over food bowls, which could have been more easily monopolised by a single individual.
This carrying of food items may reflect a more natural feeding and foraging repertoire. In their wild state, ring-tailed lemurs often forage on the ground, feeding on a range of food items including leaves and fruit, especially tamarind (Cuozzo and Sauther 2006). These large fruit items may be manipulated and carried after being found, so as to avoid conflict with other troop members (Millette et al. 2009). The increased time spent feeding may also be a better reflection of natural behavioural repertoires, as many Malagasy fruits are tough to process (Cuozzo and Sauther 2006). While cultivated food items are unlikely to reflect the low-calorie, tough foods of the wild lemur, a more challenging food presentation style may present an enriching challenge to the animals.
Chasing behaviour was the most common behaviour that might be considered aggressive in the study. Overall, chasing occurred rarely during the study, and expression of this behaviour was not associated with the type of food the lemurs had selected. This is promising, as unlike Mathy and Isbell’s (2001) study, aggression does not seem to be linked to food condition. Only one anecdotal observation of stealing occurred during the study period, Waasdorp et al’s (2021) study. Stealing was interpreted by Waasdorp et al. (2021) as an aggressive behaviour and welfare concern, so it is important to note that this behaviour did not seem to occur in the lemur group. Food sharing also did not occur in this study. However, the bachelor group structure may have reduced affiliative behaviour between individuals (Jaeggi and van Schaik 2011).
Overall, provision of whole food items appeared to provide benefits for lemurs in terms of eating and carrying, with no impact on aggression-related behaviours.
Future directions
Considering the small sample size and social grouping of lemurs in this study, there is scope to extend this research to a wider range of ring-tailed lemurs. A logical next step is to investigate the behavioural impact of food presentation in mixed-sex groups, as lemurs possess a social hierarchy in which females are dominant. Research focusing on behavioural interactions such as aggressive and affiliation would have value. Similarly, future studies could cover a wider range of lemur species, as lemurs possess a variety of different forms of feeding ecology and natural social structure. It is unlikely that one food presentation style will be effective for all species. Where possible, these future studies should take into account keeper preparation time (James et al. 2021; Griffin and Brereton 2021), and also food nutritional value.
Research should also focus on the prevalence of whole food presentation in the zoo and aquarium. It is possible that whole food feeding strategies are being used more commonly than is reported in the scientific literature. Zoos contain a wide range of species, and birds and fish are actually the best represented in terms of average number of species (Brereton and Brereton 2020). A survey investigating which species are currently being provided with whole foods, along with evidence as to whether whole food has been effective, would have value.
Finally, consideration should be given to keeper perspectives on whole food diets. Keepers may have key concerns to raise, either based on their own observations of animals, or on anecdotal evidence. While these concerns may not always be supported by evidence, it is important that these concerns are acknowledged and considered. Keepers are, after all, the people responsible for feeding animals on a daily basis, so if they have reservations about diets, they may be unwilling to adjust their feeding protocol (Hammerton et al. 2019).
On occasion, anecdotal concerns have been raised with regards to aggression implications when providing zoo-housed animals with larger food items. Contrary to these anecdotes, no aggression or welfare-based concerns arose when providing whole food items to a group of bachelor ring-tailed lemurs. Trends toward selecting whole food items over chopped items, and a non-significant increase in food intake for whole items, suggests that lemurs may actually value these larger items more than smaller pieces. Additionally, an increase in carrying behaviour suggests that lemurs are more likely to take large foods to a more secluded spot for feeding, thus reducing potential conflict over feeds. While food-based aggression was not noted for this study, this may be particularly important in mixed-sex groups where females sometimes dominate males. Use of whole food diets should be trialled across a wider range of lemur groups and species in order to improve natural feeding behaviour opportunities whilst also reducing keeper workload and food nutrient breakdown.
The authors would like to thank the team at the Animal Health and Welfare Research Centre, Sparsholt College, for their help during the project.