Carcinoma cervix is an invasive epithelial neoplasm comprised of neoplastic cells demonstrating varying degrees of squamous differentiation. Squamous cell carcinoma cervix is a frequently discerned variant of cervical carcinoma (>90%). Disease emergence is commonly observed within 40 years to 54 years. The neoplasm comprehensively (~100%) demonstrates infection with high risk subtypes of human papillomavirus (HPV) as HPV 16 and HPV 18 (HPV 16 >HPV 18) and emerges from a precursor lesion designated as high grade squamous intraepithelial lesion (HSIL). The condition is frequently encountered within individuals of low socioeconomic status and inadequate cytological screening. Disease incidence and tumour associated mortality is significantly diverse within populations of low socioeconomic status or high socioeconomic status [1,2].
Majority of cervical carcinomas emerge from squamous–columnar junction. Tumefaction exhibits a variable morphology with distinctive histological variants [1,2]. Carcinoma cervix enunciates persistent infection with high risk subtypes of human papilloma virus (HPV) denominated as subtype 6, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 or various accompanying subcategories [1,2]. Characteristically, HPV 16 engenders squamous cell carcinoma whereas HPV 18 is associated with endo–cervical adenocarcinoma. A preceding or concurrent history of high grade squamous intraepithelial lesion (HSIL) can be elicited [1,2]. Factors contributing to emergence of carcinoma cervix are young age at first intercourse, multiple sexual partners, singular contact with infected partner with consequent cervical infection, immunodeficiency due to organ transplantation, infection with human immunodeficiency virus (HIV), various immunosuppressive drugs, cigarette smoking for duration exceeding>20 years, multiparous subjects, young primigravida, extensive utilization of oral contraceptives, chronic inflammation or concurrent sexually transmitted diseases (STIs) as chlamydia, family history of carcinoma cervix or absence of circumcision of male partner.
Carcinoma cervix exemplifies enhanced prevalence of human papilloma virus (HPV) infection among adolescents and young women. Majority of infections regress spontaneously [1,2]. Although singularly inadequate, persistent infection with high risk subtypes of HPV may induce high grade squamous intraepithelial lesion and squamous cell carcinoma of cervix [1,2]. Majority of cervical carcinomas occur due to progression of precursor lesion or high grade squamous intraepithelial lesion (HSIL) wherein progression of HSIL into frank, invasive carcinoma cervix is variable and frequently delayed. Association of concurrent factors significantly enhances proportionate persistence of HPV [1,2]. High risk subtypes of HPV activate E6 and E7 oncogenes. E6 adheres to tumour suppressor gene p53 with consequent proteolytic degradation and inactivation. Consequently, p53 mediated DNA degradation may ensue with activation of apoptosis pathway [1,2]. E7 adheres to retinoblastoma (Rb) gene with subsequent displacement of transcription factors normally adherent to Rb gene along with inactivation of Rb mediated cell cycle regulation pathways. Inactivation of Rb gene engenders overexpression of p16, a tumour suppressor gene implicated in cell cycle regulation along with inhibition of cyclin dependent kinases [1,2]. Besides, p16 immunohistochemistry can be employed as a surrogate marker for detecting cervical infection with high risk HPV subtypes. Commonly, neoplasm disseminates through cervical lymphatics into regional lymph nodes or may extend directly towards vagina, uterus, parametrium, lower urinary tract or uterosacral ligaments. Distant metastases may incriminate aortic or mediastinal lymph nodes, pulmonary parenchyma, bones or uterine adnexa [1,2]. Vaccination for various high risk strains of HPV can be adopted for female subjects between 16 years to 23 years and appears as a protective phenomenon for ~12 years. Thus, two or three doses of HPV vaccination appears beneficial, contingent to age of commencement of vaccination(1,2). Generally, two doses are recommended for children and adolescents between 9 years to 14 years, irrespective of gender. Three doses appear optimal for adolescents and adults between 15 years to 26 years, irrespective of gender [1,2]. Squamous cell carcinoma cervix exhibits genomic mutations within PIK3CA gene. Mutation within KRAS or TP53 genes is infrequently observed. Besides, loss of heterozygosity (LOH) occurs within multiple chromosomal loci as 1q, 3p, 3q, 6p, 6q, 11q, 17p or 18q. Asymptomatic subjects denominate an aberrant cervical cytology. Also, vaginal bleeding, discharge or cervical tumefaction may be discerned. Tumefaction of advanced grade enunciates pain, urinary symptoms as ureteral obstruction with consequent anuria, uraemia, haematuria, frequency of micturition or vesico–vaginal fistula, gastrointestinal symptoms as tenesmus or rectovaginal fistula and lymphedema confined to lower extremities [1,2].
Upon gross examination, preliminary neoplastic transformation exhibits a reddish, friable, indurated or ulcerated lesion or elevated, granular zone. Alternatively, an exophytic, papillary, polypoid, nodular or ulcerated tumefaction may be encountered. Besides, a deep seated, infiltrative tumour mass of variable magnitude may invade circumscribing soft tissue [1,2]. Upon cytological examination, anomalous squamous epithelial cells may be observed, irrespective of specimen cellularity. Tumour diathesis with fresh or haemolysed blood and necrotic cellular debris is common. However, tumour diathesis is absent in exophytic neoplasms with <5 millimetre depth of invasion. Necrotic substance appears confined to perimeter of cellular clusters, denominated as ‘clinging diathesis’ [1,2]. Non–keratinizing variant of carcinoma cervix delineates crowded cellular groups and syncytial fragments constituted of enlarged to medium non–keratinized squamous epithelial cells with enhanced nucleo–cytoplasmic ratio. Spherical tumour nuclei with irregular outline are permeated with coarse, irregularly disseminated nuclear chromatin and macro–nucleoli. Naked nuclei may emerge. Cellular cytoplasm is scant, dense, basophilic and lacks keratinization. Keratinized singular cells are exceptional [1,2]. Keratinizing variant of carcinoma cervix exemplifies singular, dispersed squamous epithelial cells and minimal tumour diathesis. Tumour nuclei are preponderantly hyperchromatic with granular, irregular nuclear chromatin and infrequently discerned nucleoli [1,2]. Neoplastic cells are irregular, keratinized and imbued with orangeophilic cytoplasm. Squamous ‘pearls’ are frequently observed. ‘Tadpole’ shaped cells may exhibit intracytoplasmic Herxheimer spirals and keratohyaline granules. In contrast to cervical adenocarcinoma, tumour cells and nuclei appear irregular with dense cytoplasm, significantly granular nuclear chromatin and hyperchromatic nuclei [1,2].
Upon microscopy, neoplastic cells infiltrate as singular cells or configure irregular, anastomosing cellular nests. Tumefaction is circumscribed by a desmoplastic stroma imbued with an exudate of chronic inflammatory cells. Alternatively, a loosely cohesive surrounding stroma may be observed [1,2]. Tumours demonstrating superficial stromal invasion exemplify enhanced cytoplasmic eosinophilia of epithelial cells. Tumour infiltration into lymphatic and vascular spaces may ensue. Tumour grading is contingent to nuclear pleomorphism, nucleolar dimension, mitotic activity and focal necrosis whereas prognostic outcomes appear non concurrent [1,2]. Carcinoma cervix is graded as •well differentiated neoplasm composed of nests and aggregates of squamous epithelial cells of variable magnitude and outline. Keratin pearls are abundant. Tumour cells appear enlarged with well defined intercellular bridges and abundant, eosinophilic cytoplasm. Mitotic figures are occasional. Focal necrosis may be observed [1,2]. •moderately differentiated neoplasm is comprised of cords, sheets and spherical to irregular tumour cell nests of variable magnitude. Focal keratinization is observed. Tumour cells appear uniform, enlarged to medium with indistinct cellular perimeter. Mitotic activity is discernible. •poorly differentiated neoplasm represents with miniature nests, cords, sheets and singularly dispersed tumour cells. Neoplastic cells appear miniature and imbued with scanty cytoplasm with hyperchromatic nuclei. Mitotic activity is brisk. Focal keratinization is absent or exceptional [1,2]. Carcinoma cervix exhibits diverse morphologic variants as ~keratinizing variant where tumefaction demonstrates keratin pearls, abundant kerato–hyaline granules and intercellular bridges. Tumour cells display enlarged, hyperchromatic nuclei with coarse chromatin and inconspicuous nucleoli [1,2]. ~non keratinizing variant configuring sheets or nests of polygonal cells. Tumour cell nuclei appear enlarged with irregularly disseminated, coarse, granular chromatin and singular or multiple nucleoli. Mitotic activity is significant. Intercellular bridges are frequently discerned. Tumefaction is devoid of keratin pearls [1,2]. ~papillary variant composed of broad or attenuated papillae lined with multi–layered epithelium demonstrating squamous differentiation and incorporated with fibro–vascular cores, akin to high grade squamous intraepithelial lesion (HSIL). Stromal invasion may be challenging to discern upon superficial tissue samples [1,2]. ~basaloid variant is comprised of well delineated nests of immature basaloid cells, permeated with scanty cytoplasm demonstrating peripheral palisading of pleomorphic, hyperchromatic nuclei. Mitotic activity is significant. Foci of geographic or comedo–like necrosis and keratinization may occur although keratin pearls are absent. Tumefaction simulates aggressive basaloid squamous cell carcinomas delineated within diverse sites [1,2]. ~warty variant exhibits warty articulations upon superimposed surface and may resemble condyloma or bowenoid lesion of vulva upon low power magnification. Focal keratinization and koilocytic atypia may emerge [1,2]. ~verrucous variant is an extremely exceptional, inadequately denominated subtype of squamous cell carcinoma cervix. The extensively differentiated tumefaction enunciates an exophytic pattern of tumour evolution with an undulating, warty surface and appears devoid of koilocytes. Focal hyperkeratosis or parakeratosis and frond–like, acanthotic squamous epithelium may be exemplified. Neoplasm enunciates broad based, pushing foci of tumour invasion along with bulbous epithelial pegs. Neoplastic cells display abundant cytoplasm with minimal cytological atypia and infrequent mitoses [1,2]. ~squamo–transitional variant simulates squamo–transitional carcinoma of urinary bladder. Tumefaction is composed of papillae lined with multi–layered squamous epithelium with transitional differentiation. Papillae appear imbued with fibro–vascular cores. Lesion may simulate HSIL and singularly manifest transitional or squamous epithelium or epithelial concurrence may be discerned. Transitional cell metaplasia is absent [1,2]. ~lymphoepithelial–like carcinoma simulates nasopharyngeal lymphoepithelial–like carcinoma and enunciates poorly defined nests of undifferentiated, dis–cohesive squamous epithelial cells permeated with moderate cytoplasm, uniform, vesicular nuclei and conspicuous nucleoli. Indistinct cellular perimeter imparts a syncytium–like appearance to cell nests. Intercellular bridges or focal keratinization are absent. Tumour cell nests are disseminated within abundant lymphocytic aggregates. Neoplasm is associated with human papilloma virus (HPV) infection [1,2]. ~spindle cell or sarcomatoid variant is constituted of spindle–shaped cells pervaded with hyperchromatic nuclei and conspicuous nucleoli. Mitotic activity is significant and necrosis may occur. Conventional epithelioid zones appear admixed with spindle cell component. Osteoclast–like giant cells are occasionally discerned [1,2]. Exceptionally, focal mucinous differentiation, pseudo–glandular pattern secondary to acantholysis, amyloid deposits, signet ring cells, melanin granules or high grade squamous intraepithelial lesion (HSIL)–like tumour configuration can be exemplified [1,2]. Ultrastructural examination of well differentiated neoplasm exhibits well developed intracytoplasmic tono–filaments, desmosome–tonofilament complex and intercellular microvilli. Aforesaid features may be absent in poorly differentiated neoplasms [1,2].
TNM classification of carcinoma cervix is designated as Primary tumour
•TX: Primary tumour cannot be assessed •T0: No evidence of primary tumour •T1: Tumour confined to cervix •T1a: Invasive carcinoma discernible by microscopy with depth of invasion ≤5 millimetres ~T1a1: stromal invasion ≤3 millimetres ~T1a2: stromal invasion >3 millimetres and ≤5 millimetres •T1b: Invasive carcinoma with stromal invasion >5 millimetres, lesion confined to cervix uteri requiring assessment of maximum tumour diameter ~T1b1: Invasive carcinoma with stromal invasion >5 millimetres and maximum tumour diameter ≤2 centimetres ~T1b2: Invasive carcinoma with stromal invasion >5 millimetres and maximum tumour diameter >2 centimetre and ≤4 centimetre ~T1b3: Invasive carcinoma with stromal invasion >5 millimetres and >4 centimetre maximum diameter •T2: Tumour extends beyond uterus although lower one third of vagina or pelvic wall is spared •T2a: Tumour confined to upper two third of vagina without parametrial invasion ~T2a1: Invasive carcinoma ≤4 centimetre diameter ~T2a2: Invasive carcinoma>4 centimetre diameter •T2b: Tumour confined to upper two thirds of vagina with parametrial invasion although pelvic wall is spared •T3: Tumour invades lower one third of vagina, extends to pelvic wall and engenders hydronephrosis or non functioning kidney ~T3a: Tumour invades lower one third of vagina with absent invasion to pelvic wall ~T3b:Tumour extends to pelvic wall with hydronephrosis or non functioning kidney •T4: Tumour incriminates mucosa of urinary bladder or rectum or extends to adjacent organs which can be confirmed upon microscopic examination [2,3].
Regional lymph nodes •NX: Regional lymph nodes cannot be assessed •N0: Regional lymph node metastasis absent ~N0(i+): Isolated tumour cells confined to regional lymph nodes ≤0.2 millimetres or singular cells or clusters of neoplastic cells ≤200 cells within a singular lymph node •N1: Regional lymph node metastasis into pelvic lymph nodes ~N1mi: Regional lymph node metastasis >0.2 millimetres and ≤2.0 millimetres diameter into pelvic lymph nodes ~N1a:Regional lymph node metastasis >2.0 millimetre diameter into pelvic lymph nodes •N2: Regional lymph node metastasis into para–aortic lymph nodes along with or devoid of pelvic lymph node metastasis ~N2mi: Regional lymph node metastasis >0.2 millimetres and ≤2.0 millimetres diameter into para–aortic lymph nodes along with or devoid of pelvic lymph node metastasis ~N2a: Regional lymph node metastasis >2.0 millimetre diameter into para–aortic lymph nodes along with or devoid of pelvic lymph node metastasis(2,3). Additionally ●Nf: Regional lymph node metastasis identified by fine needle aspiration or core needle biopsy. ●Nsn: Regional lymph node metastasis identified by sentinel lymph node biopsy. Regional lymph node metastasis is categorized contingent to magnitude of metastases into a) individual tumour cells (ITCs) demonstrating <0.2 millimetre diameter b) micro–metastasis exhibiting 0.2 millimetre to 2 millimetre diameter c) macro–metastasis delineating >2 millimetre diameter [2,3].
Distant metastasis •M0: Distant metastasis absent •cM1: Distant metastasis into pulmonary or hepatic parenchyma, bone, inguinal lymph nodes and intraperitoneal disease. •pM1: Distant metastasis into pulmonary or hepatic parenchyma, bone, inguinal lymph nodes and intraperitoneal disease which can be confirmed upon microscopic examination [2,3].
Squamous cell carcinoma cervix is immune reactive to pancytokeratin, CK5/6, EMA, p63, p40, p16, PDL1, CEA, CK7, PAX8 or GATA3. Tumefaction is immune non–reactive to inhibin, MELCAM, HPL, Napsin A, HNF–1B, p53 or CK20 [3,4] (Figure 1 & 2).
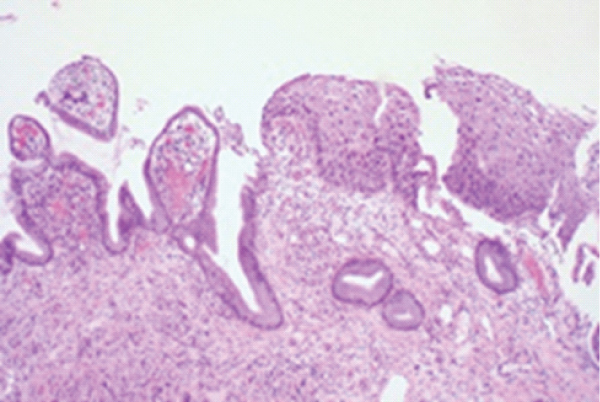
Figure 1: Carcinoma cervix (LSIL) demonstrating nuclear hyperplasia, hyperchromasia and atypia of basal epithelium superimposed upon unremarkable endocervical stroma. Mitotic activity is minimal. Stromal invasion is absent [5].

Figure 2: Carcinoma cervix (HSIL) delineating nuclear hyperplasia, hyperchromatic nuclei and full thickness atypia with few mitotic figures [6].
Carcinoma cervix requires segregation from neoplasms such as clear cell carcinoma, adenoid basal carcinoma, adenoid cystic carcinoma, small cell neuroendocrine carcinoma, carcinosarcoma, epithelioid trophoblastic tumour, immature squamous metaplasia, placental site nodule, HSIL of endo–cervical glands, sexually transmitted infections, cervical fibroid, endometriosis or cervical polyps [3,4].
Carcinoma cervix can be appropriately discerned upon histological examination of surgical tissue samples. Neoplasms of stage IB may be discerned upon radiological examination. Ultrasonography demonstrates a hypoechoic, heterogeneous tumefaction [3,4]. Colour Doppler enunciates a neoplasm with increased vascularity. Computerized tomography (CT) is optimal for assessing regional lymph node enlargement and metastatic disease [3,4]. Positron emission tomography (PET–CT) can be employed to exclude regional lymph node and distant metastases. Magnetic resonance imaging (MRI) is a preferred imaging modality adopted to assess extent of primary tumour. Tumefaction represents as a mass lesion with enhanced signal as compared to low signal engendered by circumscribing cervical stroma [3,4].
Contingent to disease stage, International Federation of Obstetrics and Gynaecology (FIGO) and National Comprehensive Cancer Network (NCCN) guidelines allocate definitive therapeutic regimens for carcinoma cervix. Conization or loop electrosurgical excision is a recommended procedure for treating low grade, stage (IA) neoplasms. Tumours of advanced grade are optimally subjected to radical trachelectomy or radical hysterectomy with mapping of sentinel lymph node or pelvic lymph node dissection along with or in the absence of radiotherapy [3,4]. Advanced, high grade neoplasms can be managed with radiotherapy and platinum based chemotherapy or pelvic exenteration [3,4]. Factors contributing to inferior prognostic outcomes are age of incriminated subject, tumour stage, depth of tumour invasion, disease volume and occurrence of lymphatic and vascular invasion [3,4]. Lympho–epithelial variant and verrucous variant exhibit superior prognostic outcomes. Neoplasms exhibiting decimated CD4+ lymphocyte count, especially within seropositive individuals infected with human immunodeficiency virus (HIV) enunciate an unfavourable prognosis [3,4]. Stage IA demonstrates an overall 5 year disease free survival of ~95% and stage IV appears at ~15% [3,4].
None.
The author declares that there is no conflict of interest.