Background: Accumulating evidence suggests overexpression of Eph receptors is associated with malignant human gliomas. Inhibiting interactions of Eph receptors with their ephrin ligands may improve clinical outcomes in glioma patients. The present study investigated the potential of cannabinoids to bind Eph receptors and block Eph/ephrin interactions.
Methods: Twelve major cannabinoids were computationally docked with ligand binding domains from six glioma–associated Eph receptors through Auto Dock Vina to measure their potential binding affinities. The molecular structures and residue interactions of the most favorable poses for each receptor binding domain were further visually examined.
Results: Cannabichromene (CBC) exhibited the most favorable binding with EphA2, EphA3, and EphB4 receptor ligand binding domains while tetrahydrocannabinol (THC) was predicted to bind favorably with EphB2 and EphB3 receptor ligand binding domains. EphA4 showed the best potential binding affinity with cannabidivarin (CBDV). Further analysis revealed that these cannabinoids bind to specific locations on Eph receptors required for Eph/ephrin interactions.
Conclusion: The findings suggest that certain cannabinoids can effectively bind to hydrophobic pockets required for ephrin binding and thereby be used to block subsequent Eph/ephrin interactions.
Keywords: Cannabinoids; Eph receptor; Ephrin; Glioma; Docking.
Abbreviations:LGG: Low Grade Glioma; HGG: High Grade Glioma; TSA: Tumor–Specific Antigens; TAA: Tumor–Associated Antigens; CBC: Cannabichromene; CBDV: Cannabidivarin; THC: Delta–9–tetrahydrocannabinol.
Glioma is a common type of tumor arising from glial supportive cells of the central nervous system and are classified into grades I to IV based on clinical and histological criteria [1–3]. Grade I gliomas are frequently curable with surgical resection and occur primarily in children whereas grade II–IV gliomas are often seen in adults [3]. Adult grade II tumors, also known as low grade gliomas (LGG), commonly include astrocytomas, mixed oligo–astrocytomas, and oligodendrogliomas. The majority of grade II lesions progress to high–grade III/IV gliomas (HGG). The grade IV gliomas are also termed glioblastomas [3]. Since surgical resection of gliomas often presents with difficulties arising from tumor infiltration and proximity to critical vessels and nerves, molecular targeting of glioma cells is becoming an attractive therapeutic avenue.
Numerous tumor–specific and tumor–associated antigens (TSA and TAA) are continuously being discovered, allowing for development of glioma–specific therapies that spare non–diseased CNS cells [4,5]. Many of these antigens have been found on the tumor cell surface as plasma membrane receptors [6,7]. Strategies to target these TSA and TAA involve using antibodies conjugated with cytotoxic drugs, compounds that block ligand–receptor interactions, and liposomes containing drugs that specifically target gliomas [8,9]. One such TAA include Eph receptors in the Eph/Ephrin receptor system in gliomas, which have been found to play critical roles in tumorigenesis [10]. Eph receptors are the largest family of receptor tyrosine kinases and are divided in two subgroups, EphA and EphB, based on the type of ligands (ephrins) they bind [11–13]. Interactions between the appropriate ephrin ligand and Eph receptor activate transducer signaling cascades involved in various biological functions, including nervous system development and angiogenesis.
A number of studies have demonstrated the overexpression of Eph receptors in human malignant gliomas. The EphA2 and EphA3 receptors is overexpressed in ~60% of glioblastomas and is associated with poor prognosis, with EphA3 knockdown tumors exhibiting decreased tumorigenic potential [14–17]. EphA4 receptor overexpression increases cell proliferation through the FGFR1 signaling pathway [10,18]. EphB2, EphB3, and EphB4 of the EphB subfamily have also been documented to be overexpressed in HGG compared to normal brain [19]. Taken together, these studies suggest mitigating these Eph/ephrin interactions may improve clinical outcomes in glioma patients. Indeed, numerous small molecules are currently being screened for targeting Eph receptors [20].
Cannabinoids, unique compounds produced from the hemp plant Cannabis sativa L., are also actively being studied as therapeutic agents in oncology. Among their possible applications, cannabinoids have been known to exert palliative effects in cancer patients [21]. Additionally, cannabinoids have been shown to inhibit growth and angiogenesis of gliomas in animal models [22,25]. Although these naturally occurring compounds have been gaining notoriety for putative medicinal use, there have been few studies exploring their molecular potential in the treatment of aggressive and rare cancers. To address this gap, the current study sought to explore the potential of major cannabinoids as inhibitors of Eph/ephrin interactions.
Since the extracellular ligand binding domains of Eph receptors have been characterized as the minimal regions required for high affinity interactions, the cannabinoids were tested for their ability to bind to these regions and potentially block ephrin attachment [26]. Therefore, through in–silico molecular docking, the present study tested the interactions of major cannabinoids with ligand binding domains of glioma–associated Eph receptors. By analyzing the potential binding affinities and residue interactions, the likelihood of successful cannabinoid–Eph receptor complex formations was evaluated. The analysis of the most favorable binding interactions shown here provides a basis for demonstrating the pharmacological potential of using cannabinoids to target the Eph/Ephrin system in gliomas.
The crystal structures for ligand binding domains of Eph receptors used in this study were retrieved from Protein Data Bank (PDB). Ligand binding domains for EphA2, EphA3, and EphA4 receptors were downloaded from PDB ID 6NK2, 4L0P, and 4W50, respectively. Ligand binding domains for EphB2, EphB3, and EphB4 receptors were downloaded from PDB ID 2QBX, 3P1I, and 2BBA, respectively.
To compare the potential binding affinities of cannabinoid–Eph receptor interactions to native ephrin–Eph receptor interactions, computational docking was also performed with the preferred ephrin ligands for each Eph receptor. The receptor binding domains of these ephrin ligands, Ephrin A1, Ephrin A5, Ephrin A4, and Ephrin B2, were downloaded from PDB ID 3CZU, 1SHX, 2WO2, and 1KGY respectively.
The cannabinoid ligand structures (shown in Figure 1) were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/) as three–dimensional SDF files. Receptor and ligand files were converted to PDBQT format using the Open Babel package [27].

Figure 1: Structures of cannabinoids evaluated for binding affinities with Eph receptor ligand binding domains.
AutoDock Vina was used to perform molecular docking analyses [28]. Preparation of grid box was implemented in PyMol (https://pymol.org/2/#page–top). Grid spacing was set to 1.0 Å and exhaustiveness was set at 10. Grid size was set to cover entire ligand binding domain in order to analyze all potential binding sites. The results less than 2.0 Å in positional root–mean–square deviation (RMSD) were clustered together and represented by the conformation result with the most favorable binding affinity. The binding pose with the most favorable potential binding affinity for each cannabinoid was extracted for further visual analysis in PyMol and LigPlot+ v.2.2 (https://www.ebi.ac.uk/thornton–srv/software/LigPlus/).
Detailed analysis of the docking simulations revealed that cannabinoids, in general, interacted more favorably with the EphB class of receptors compared to EphA receptors (shown in Figure 2). The potential binding affinities ranged from –3.7 to –8.3 kcal/mol. The potential binding affinities were also comparable to those calculated for the preferred ephrin ligands (shown in Table 1).

Figure 2: Heatmap of binding affinities for cannabinoids (y–axis) and Eph receptor ligand binding domains (x–axis). Colour legend indicates binding affinity free energy in kcal/mol.
Table 1: Predicted binding affinities of Eph receptors and their preferred Ephrin ligands.
Eph Receptor |
Preferred Ligand |
Potential Binding Affinity (kcal/mol) |
EphA2 |
Ephrin–A1 |
–8.2 |
EphA3 |
Ephrin–A5 |
–7.6 |
EphA4 |
Ephrin–A4 |
–8.4 |
EphB2 |
Ephrin–B2 |
–7.3 |
EphB3 |
Ephrin–B2 |
–7.5 |
EphB4 |
Ephrin–B2 |
–7.5 |
EphA2
The most favorable potential binding interaction for EphA2 occurred with cannabichromene (CBC) near the E–F loop of the receptor. The binding affinity was reported to be –6.5 kcal/mol. About 23 hydrophobic interactions were identified within the complex with amino acids Cys188, Val189, Ala190, Ser68, Val69, Cys70, Asp53, and Met55 (shown in Figure 3).

Figure 3: a) Overall structure of predicted binding pose of cannabichromene (CBC) with EphA2 receptor ligand binding domain. b) 2–D representation of CBC–EphA2 binding domain complex with hydrophobic contacts shown by arc spokes.
EphA3
CBC was also predicted to result in the most favorable affinity with the binding domain of the EphA3 receptor. The binding pose was predicted near the H–J loop. The interactions contributing to the potential binding affinity of –6.6 kcal/mol include hydrophobic interactions between cannabichromene and amino acids Ile168, Arg169, Asp144, Ile143, Glu170, Gly172, Tyr124, and Lys142 (shown in Figure 4).

Figure 4: a) Overall structure of predicted binding pose of cannabichromene (CBC) with EphA3 receptor ligand binding domain. b) 2–D representation of CBC–EphA3 binding domain complex with hydrophobic contacts shown by arc spokes.
EphA4
Cannabidivarin (CBDV), a cannabidiol homolog with a propyl chain, exhibited the best potential binding affinity with EphA4 receptor ligand binding domain. The free energy of the interaction was –5.8 kcal/mol with binding localized near the G–H loop. The binding affinity was attributed to hydrophobic interactions with Arg171, Ile145, Lys144, Tyr126, Val173, Asp172, Arg96, and Gly174 (shown in Figure 5).
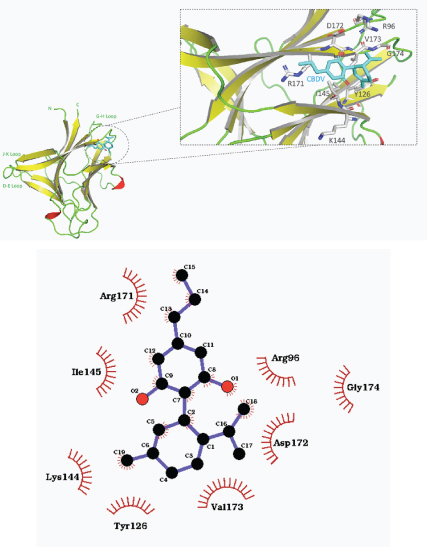
Figure 5: a) Overall structure of predicted binding pose of cannabidivarin (CBDV) with EphA4 receptor ligand binding domain. b) 2–D representation of CBDV–EphA4 binding domain complex with hydrophobic contacts shown by arc spokes.
EphB2
Docking with delta–9–tetrahydrocannabinol (THC) yielded the most favorable pose relative to the other cannabinoids tested with the EphB2 receptor binding domain. THC was docked near the J–K and G–H loop with a predicted binding affinity of –7.6 kcal/mol (shown in Figure 6). Hydrophobic interactions dominated the molecular binding, with hydrophobic interactions predicted with Tyr57, ile196, Gly56, Thr66, Ser194, Gln68, Val102, Lys166, Val164, Arg103, Ser101, Met165, and Leu160.
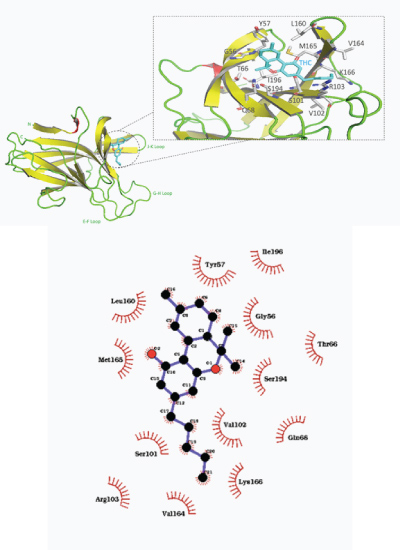
Figure 6: a) Overall structure of predicted binding pose of tetrahydrocannabinol (THC) with EphB2 receptor ligand binding domain. b) 2–D representation of THC–EphB2 binding domain complex with hydrophobic contacts shown by arc spokes.
EphB3
THC also showed the most favorable binding pose in close proximity with J–K and G–H loops of the EphB3 binding domain, with a predicted binding affinity free energy of –8.3 kcal/mol (shown in Figure 7). Hydrophobic interactions were noticed between THC and amino acids including Gly61, Ser60, Val80, Met200, Ser201, Cys199, and Gln79.
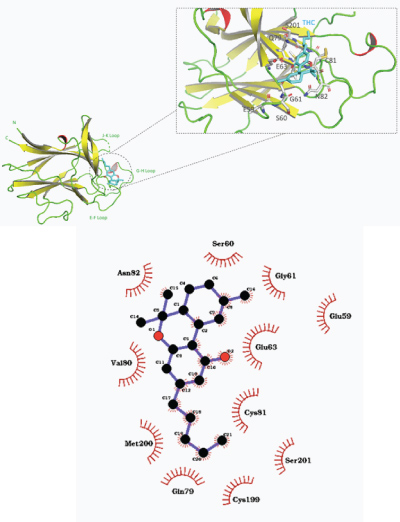
Figure 7: a) Overall structure of predicted binding pose of tetrahydrocannabinol (THC) with EphB3 receptor ligand binding domain. b) 2–D representation of THC–EphB3 binding domain complex with hydrophobic contacts shown by arc spokes.
EphB4
CBC showed the most favorable predicted binding affinity of –8.2 kcal/mol with the EphB4 ligand binding domain. Specifically, the predicted pose exhibits CBC bound near the J–K and G–H loop inside the hydrophobic ligand binding cavity, with hydrophobic interactions with Leu95, Ala155, Leu100, Pro151, and Gly157 (shown in Figure 8).
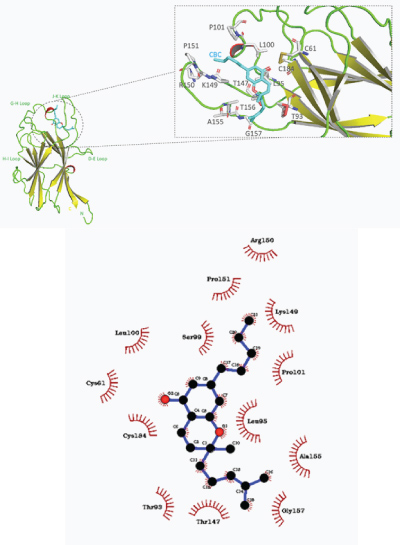
Figure 8: a) Overall structure of predicted binding pose of cannabichromene (CBC) with EphB4 receptor ligand binding domain. b) 2–D representation of CBC–EphB4 binding domain complex with hydrophobic contacts shown by arc spokes.
Presented herein were computational binding analyses of major cannabinoids to Eph receptors previously reported to be associated with gliomas. It was seen that the cannabinoids displayed predicted binding affinities comparable to preferred Eph receptor–ephrin ligand interactions, notably the Eph B receptor– cannabinoid predicted affinities. Further analysis showed that hydrophobic interactions dominated each of the most favorable cannabinoid–Eph receptor binding poses. These binding patterns concur with the hydrophobic nature of cannabinoids. Specifically, the cannabinoids with the most favorable interactions were predicted to bind near the G–H and J–K loops of the Eph receptors. Indeed, in high affinity interfaces, the G–H loop of respective ephrin ligands insert into the hydrophobic ligand binding sites formed by the D–E, G–H, and J–K loops of the Eph receptors [26,29]. Since the cannabinoids evaluated in the present study have been shown to interact favorably with these regions, and show potential binding affinities comparable to preferred ephrin ligands, they may be able to indeed block ephrin ligands from binding to the hydrophobic pocket created by these flexible loops.
Remarkably, CBC exhibited the best predicted binding affinities for three of the six Eph receptor binding domains tested, namely EphA2, EphA3, and EphB4. CBC displayed the greatest affinity binding pose near the J–K and D–E loops in the hydrophobic cleft of EphB4, similar to the binding interfaces of previously reported EphB4/ephrin antagonist peptide complexes [30]. THC showed the best binding affinity for binding domains of EphB2 and EphB3 receptors out of the twelve cannabinoids evaluated. It was noticed that THC binds to the same cleft on EphB2 as the SNEW peptide antagonist (SNEWIQPRLPQH) which was shown to effectively compete with ephrin ligands for receptor binding [31].
It should be noted that EphB receptors generally demonstrated the most favorable potential binding affinities compared to EphA receptors in the present study. Although all Eph receptor contain highly conserved structural features and domains, the primary sequence differences between the receptor subclasses that exists in a region of the ligand binding domain may contribute to the differential affinities seen herein [32]. Given the high structural similarity of EphA and EphB receptors, future studies should also consider the spatial and temporal distribution patterns of Eph receptors, as the microenvironment might contribute to binding configurations.
The binding interactions shown here demonstrate potential for cannabinoids to inhibit Eph/ephrin interactions by blocking the hydrophobic ephrin ligand binding sites on Eph receptors. Although the present study was limited to in silico predictions, the preliminary data should accelerate efforts for using these naturally occurring compounds in glioma treatment. Further in vitro and in vivo characterizations are warranted to confirm the potential of these cannabinoids to inhibit ephrin–Eph receptor interactions.
Ethical Approval and Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Availability of supporting data
All data used in the present study is provided through the sources stated in the manuscript.
Competing interests
The author has no competing interests to declare.
Funding
Not applicable.
Author Contributions
SB formulated design and analyzed data. Results were interpreted by SB and MD. Manuscript was written and edited by SB, MD, and BL. All authors read and approved final manuscript.
Acknowledgements
Not applicable.