Hashimoto’s thyroiditis is an autoimmune disorder demonstrating thyroid enlargement or goitre associated with elevated circulating anti–thyroid peroxidase and anti–thyroglobulin antibodies. Painless, gradually progressive failure of thyroid function and autoimmune damage to thyroid parenchyma is observed. Diffuse, symmetric enlargement of thyroid gland enunciates an extensive infiltrate of mature lymphocytes with configuration of germinal centres. In addition, atrophic thyroid follicles appear layered by abundant Hürthle cells or oncocytes.
Initially scripted by Hakaru Hashimoto in 1912, Hashimoto’s thyroiditis is an autoimmune disorder demonstrating thyroid enlargement or goitre associated with elevated circulating anti–thyroid peroxidase and anti–thyroglobulin antibodies [1]. Commonly, the condition induces sporadic goitre within paediatric population residing in zones with abundant iodine. Additionally scripted as Hashimoto’s disease, struma lymphomatosa, chronic lymphocytic thyroiditis or chronic autoimmune thyroiditis, the disorder may enunciate ’Hashitoxicosis’ constituted of amalgamated features of Hashimoto’s thyroiditis and Graves’ disease [2,3]. Hashimoto’s thyroiditis can concur with diverse autoimmune disorders as systemic lupus erythematosus, rheumatoid arthritis, Sjögren’s syndrome, pernicious anaemia, type 1 diabetes mellitus, Graves’ disease, chronic active hepatitis, adrenal insufficiency, MALT lymphoma of gastrointestinal tract and associated B cell lymphomas [2,3].
Majority (~95%) of instances are discerned within females of up to 65 years. Subjects with associated Down’s syndrome exhibit lack of female preponderance. Caucasians are predominantly implicated and familial clustering may ensue [2,3]. Clinically, a painless, gradually progressive failure of thyroid function emerges in incriminated adults. Autoimmune damage to thyroid parenchyma may demonstrate initial, transient hyperthyroidism. Incriminated children exhibit variable hypothyroidism followed by euthyroid state. Thus, monitoring of thyroid function is prerequisite to appropriate disease discernment [2,3].
Hashimoto’s thyroiditis exemplifies distinctive manifestation of histocompatibility antigens as HLA–DR5 associated with goitre and HLA–DR3 concurring with atrophic form [2,3]. Hashimoto’s thyroiditis may be associated with or evolve into well differentiated thyroid carcinoma or thyroid lymphoma [2,3]. Cytological examination exhibits a moderately cellular aspirate composed of aggregates of oncocytic cells incorporated with abundant, fine, granular cytoplasm and variably pleomorphic enlarged, hyperchromatic nuclei. Oncocytes are admixed with mature lymphocytes. Besides, follicular epithelial cells, plasma cells, macrophages or neutrophils may be discerned [2,3].
Lymphocytic infiltration of thyroid parenchyma is graded from zero to three+, contingent to intensity of chronic inflammatory cell infiltrate as mature lymphocytes. However, previously mentioned grading appears non–concurrent to diverse clinical manifestations or prognostic outcomes. Upon gross examination, diffuse, symmetric enlargement of thyroid gland is observed. Occasionally, thyroid gland is nodular and asymmetrically enlarged [2,3]. Glandular atrophy may occur. The gland is encapsulated and gross weight varies from 25 grams to 250 grams. Pyramidal lobe may be prominent. Thyroid gland may adhere to although can be detached from surrounding structures and soft tissue. Cut surface is tan to yellow with enhanced interlobular fibrotic tissue and simulates lymph node. Focal necrosis or calcification is absent [2,3]. Upon microscopy, an extensive infiltrate of mature lymphocytes with configuration of germinal centres is observed. Invading lymphocytes are predominantly comprised of T lymphocytes and appear admixed with polyclonal plasma cells. Occasionally, lesion may be nodular [2,3]. Thyroid epithelial cells appear imbued with enlarged or overlapping nuclei. Few nuclei may demonstrate partial nuclear clearing. Additionally, enlarged squamous cell nests, hyperplastic lymphoid follicles and foci of ductal metaplasia may be discerned. Giant cell reaction may ensue [2,3].
Atrophic thyroid follicles abundantly coated with Hürthle cells or oncocytes are observed. Colloid is appropriately maintained. Fibrosis is enhanced, intra–parenchymal and confined by thyroid capsule. Foci of squamous metaplasia emerging within follicular epithelium may require distinction from solid epithelial cell nests [2,3]. Tumefaction may exhibit preliminary, focal, oxyphilic metaplasia of follicular epithelial cells. Nodular tumour configuration may ensue. Thyroid parenchyma is gradually replaced with aforesaid cellular content [2,3]. Cells of Hashimoto’s thyroiditis appear immune reactive to high molecular weight keratin or p63. Elevated kappa/lambda ratio occurs due to enhanced production of kappa light chains secreted by plasma cells [4,5] .
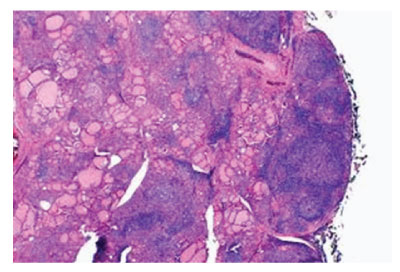
Figure 1: Hashimoto’s thyroiditis demonstrating a polyclonal inflammatory exudate of lymphocytes and plasma cells with configuration of germinal centres traversed with fibrous tissue and few intervening thyroid follicles [5].
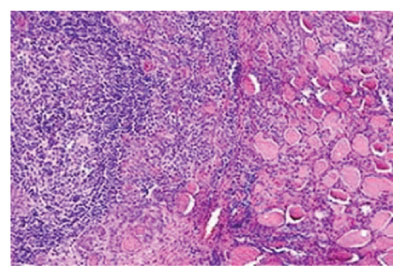
Figure 2: Hashimoto’s thyroiditis delineating lymphocytic infiltrate with prominent germinal centres admixed with thyroid follicles imbued with colloid [6].
Admixed infiltrate of T lymphocytes and B lymphocytes appears polyclonal [4,5]. Ultrastructural examination exhibits oncocytic cells incorporated with enlarged mitochondria and decimated cellular organelles [4,5]. Hashimoto’s thyroiditis requires segregation from conditions such as lithium ingestion, Hürthle cell neoplasms, MALT lymphoma, papillary thyroid carcinoma, euthyroid sick syndrome, toxic nodular goitre, Graves’ disease or diffuse toxic goitre, nontoxic goitre, hypopituitarism, poly–glandular autoimmune syndrome type 1 or poly–glandular autoimmune syndrome type 2 [4,5]. Hashimoto’s thyroiditis is associated with autoantibodies as anti TSH antibodies which appear specific for Hashimoto’s thyroiditis and Graves’ disease, anti–thyroglobulin antibodies, anti–thyroid peroxidase or anti–microsomal antibodies and exceptionally, an anti–iodine transporter [4,5]. Generally, therapeutic intervention of Hashimoto’s thyroiditis remains unnecessary. However, thyroid hormone can be administered for treating the hypothyroid stage [4,5]. Upon thyroidectomy, carcinoma thyroid can be commonly discerned. Subtotal thyroidectomy can be suitably adopted to relieve mass effect of thyroid nodule. Exceptionally, the condition progresses to lymphoma [4,5,7].
Hashimoto’s thyroiditis requires segregation from conditions such as lithium ingestion, Hürthle cell neoplasms, MALT lymphoma, papillary thyroid carcinoma, euthyroid sick syndrome, toxic nodular goitre, Graves’ disease or diffuse toxic goitre, nontoxic goitre, hypopituitarism, poly–glandular autoimmune syndrome type 1 or poly–glandular autoimmune syndrome type 2. Hashimoto’s thyroiditis is associated with anti TSH antibodies which are specific for Hashimoto’s thyroiditis and Graves’ disease, anti–thyroglobulin antibodies, anti–thyroid peroxidase or anti–microsomal antibodies. Subtotal thyroidectomy can be suitably adopted to relieve mass effect of thyroid nodule.
None.
Author declares that there is no Conflict of interest.