Herein, we present the case of a 55-year-old female who presented with subarachnoid hemorrhage (SAH) due to rupture of a posterior communicating artery infundibular aneurysm. The patient was treated during the acute phase of SAH via balloon-assisted coil embolization. The patient recovered without any neurologic sequalae. There is a dearth of literature describing the use of an endovascular approach for the treatment of ruptured intracranial infundibula or infundibular aneurysms. We propose the use of balloon assisted coil embolization as a viable therapeutic option for the treatment of ruptured intracranial infundibular aneurysms and present a detailed review of the literature.
Keywords: Subarachnoid Hemorrhage; Infundibulum; Balloon Assisted; Treatment.
Intracranial infundibula most often arise from the posterior communicating artery (PcoA) and are defined as a vascular outgrowth characterized by a round or conical shape. They have a maximal diameter not exceeding three millimeters and are without an aneurysmal neck. With an infundibulum, the PcoA typically arises from its apex [1]. The idea that intracranial infundibula represent a normal anatomic variant has been challenged in recent years by several reports documenting infundibulum progression. This can lead to true aneurysm and subarachnoid hemorrhage (SAH) following infundibular rupture or in cranial nerve deficit [2]. Recent evidence indicates that aneurysms form de novo from infundibulum [3]. The optimal approach for managing this entity has yet to be elucidated, but with the addition of endovascular intervention to the cerebrovascular neurosurgeon’s armamentarium, novel minimally invasive techniques may prove to be the ideal treatment for these patients. To date, there have been three publications describing endovascular obliteration of an infundibulum or infundibular aneurysm [4]. We present the first case describing the use of balloon-assisted coil embolization for the treatment of a ruptured PcoA infundibular aneurysm in a patient during the acute phase of SAH.
A 55-year-old woman with a family history of ruptured cerebral aneurysm complained of the worst headache of her life. She presented as a Hunt Hess 3 and Fisher 4 SAH with symptomatic hydrocephalus, requiring an external ventricular drain. Computed tomographic angiography demonstrated what appeared to be a ruptured posterior communicating artery aneurysm. Cerebral angiography demonstrated a ruptured PcoA infundibulum. On 3-D rotational angiography, an aneurysm measuring 1.7 x 2.4 mm was noted off the right PcoA infundibulum with a daughter lobe on the dome, suggestive of a rupture point (Fig. 1 and 2). Balloonassisted coil embolization was performed successfully, with no residual aneurysm, and the PcoA remained patent (Fig. 3). During her hospitalization she developed Takotsubo cardiomyopathy and symptomatic vasospasm, both of which were managed medically and resolved at discharged without any permanent sequelae. The patient was neurologically intact at time of discharge to an acute rehabilitation facility.
The patient remained neurologically intact at ten month follow up. Repeat digital subtraction angiogram (DSA) demonstrated a coil mass at the previously treated Murphy’s tit of the right PcoA infundibulum. This is important to note when assessing infundibulum as de novo aneurysms can develop at Murphy’s tit. In addition, a growth measuring 3.7 x 2.4 mm was noted off the right PcoA infundibulum, suspicious for a new aneurysm or possible persistent residual aneurysmal cavity following previous endovascular embolization. This showed the dynamic nature of infundibulum growth.
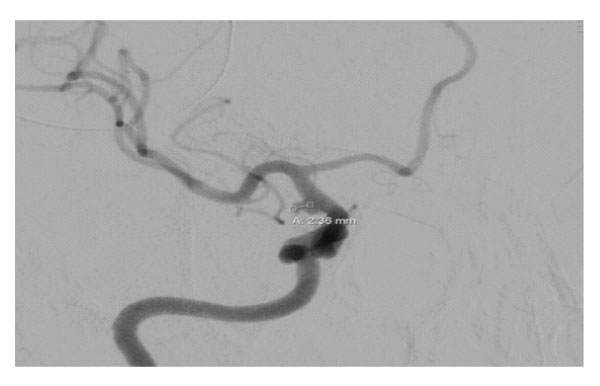
Figure 1: : Pre-operative oblique view cerebral angiogram demonstrating posterior communicating artery infundibulum.

Figure 3: Post-coiling oblique view cerebral angiogram demonstrating secured posterior communicating artery infundibulum.
The beginning of modern-day vascular neurosurgery essentially dates to 1937 when Walter Dandy successfully executed the first premeditated surgical clipping of an intracranial aneurysm. In 1944, Dandy published his findings in the analysis of 133 aneurysms in a seminal work titled Intracranial aneurysms—the first book detailing a large series of intracranial aneurysms, thereby setting the stage for vascular neurosurgery [5]. The treatment of intracranial aneurysms has been, and will continue to be, a mainstay of the cerebrovascular neurosurgeon. Recently, there has been an exponential growth in the knowledge of the pathophysiology of intracranial aneurysms, and with that, a paradigm shift toward endovascular techniques from open surgical approaches for the treatment of these abnormal vessels [6].
There is significant controversy in the neurosurgical literature on the natural history, and best management practices, of intracranial infundibula and infundibular aneurysms. The criteria for defining a vascular outgrowth as a PCoA infundibulum are as follows: round or conical shape, less than 3 millimeters in maximum diameter, without aneurysmal neck, and with a PCoA arising from its apex [7]. Infundibula of the intracranial vessels have previously been described as a normal anatomic variation in up to 25% of the population; comparatively, the estimated prevalence of intracranial aneurysms in the international population is 6.5% [9] To date, there have been 15 documented cases of infundibular progression to frank aneurysm, and 14 documented cases of SAH due to ruptured infundibulum [8] In 1998, Marshman et al. published the 11th case of SAH secondary to infundibular rupture, and suggested that in certain instances infundibulum may be prone to aneurysmal development and rupture. Proposed predisposing factors for infundibulum to aneurysm progression include: 1) the presence of aneurysmal histology with true disruption of the integrity of the internal elastic lamina 2) altered hemodynamics through the PcoA secondary to contralateral carotid artery ligation 3) atypical bulges present on an infundibulum, and 4) an association with increased size of the PcoA.
In 2002, Martins et al. published a case of ruptured PCoA infundibular aneurysm treated by open surgery with a unilateral craniotomy approach for SAH in a patient with multiple, and bilateral, aneurysms. Interestingly, an infundibulum, devoid of aneurysmal change, was documented 11 months prior during treatment of previous SAH. This rapid progression of infundibulum to aneurysm is inconsistent with the proposed dogma that the process occurs over a time period of about seven years.9 The rate of infundibulum progression to overt aneurysm remains uncertain, and in the absence of a study utilizing serial angiograms to depict aneurysmal development, an accurate prediction cannot be made with reasonable confidence [10]. There may be a predisposition to infundibular development in those individuals harboring a yet undetermined genetic variant associated with familial intracranial aneurysms. The increased incidence of infundibular development in individuals with a family history of intracranial aneurysms, and in certain disease states (ie. Autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome, aortic coarctation, and Alagille syndrome) support the notion of a genetic component influencing infundibular development. Moreover, in the familial aneurysm population, it is thought that the incidence of bilateral infundibula is much greater than in the general population [11]. Furthermore, Punt documented an increased incidence of infundibula in patient populations with high incidence of internal carotid artery aneurysms and multiple aneurysms, thereby suggesting that the pathogenesis underlying aneurysmal development predisposes for the presence of a synchronous infundibulum [12]. Patients with inherited cardiovascular disease, like Alagille syndrome, may also be at an increased risk of developing infundibula, with progression into aneurysms, and subsequent rupture. Cowan et al. reported the 13th case of ruptured PCoA aneurysm in a patient with Alagille syndrome [13]. Five years earlier, a PCoA infundibulum was noted when the patient was treated via open craniotomy and surgical clipping for SAH due to rupture of a basilar tip aneurysm. The patient was, again, treated with surgical clipping of the PCoA aneurysm that had developed at the site of the PCoA infundibulum noted five years earlier. At three-month follow-up, the patient exhibited no signs of neurological dysfunction [13].
Despite several reported cases of PcoA infundibular rupture, and rupture following infundibular progression to overt aneurysm, there have only been three publications describing endovascular obliteration of an infundibulum or infundibular aneurysm [14]. Moreover, a search of the literature revealed no reports of infundibular aneurysm recurrence after initial treatment whether following open surgical clipping via craniotomy or endovascular obliteration. In 2010, Yu et al. described the first case of ruptured PcoA infundibulum successfully treated via an endovascular approach. The authors successfully performed stent assisted coil embolization in a 35-year-old male patient, 27 days after presenting with symptomatic SAH. The patient was initially managed with conservative medical therapy until repeat head CT revealed resorption of subarachnoid blood. The patient received a one-week regiment of aspirin and clopidogrel prior to endovascular coil embolization. At one-year follow up, cerebral angiography revealed no evidence of residual infundibulum of the PcoA [15].
Fischer et al. reported a case of successful stent-assisted coil occlusion of a PcoA aneurysm previously identified as a PcoA infundibulum in a patient who was previously treated twice using endovascular coil embolization for saccular aneurysms of the right V4 segment of the vertebral artery and the vertebral artery junction. At follow up 16 months after PcoA aneurysm stent-assisted coil occlusion, digital subtraction angiography demonstrated complete occlusion of the aneurysm [5].
In 2014, Kameda-Smith et al. described the embolization of a right ICA aneurysm using a Pipeline flow-diverting device with concomitant placement of nylon fiber coils. In this case, a 66-year-old female suffered SAH with bleeding emanating from an aneurysm in the distal right ICA, just distal to a right PCoA infundibulum. The Pipeline flow-diverting device was placed with the intention of treating the ICA aneurysm, but incidentally extended across the right PCoA infundibulum. DSA 12 days following endovascular aneurysm embolization showed full occlusion of the aneurysm, and by three months remodelling of the right PCoA infundibulum into a normal origin. This case represents the first report of PcoA infundibular regression following placement of a pipeline flow-diverting device [16].
To our knowledge, our case is the first reported successful embolization of a PcoA infundibular aneurysm using a balloon-assisted coiling technique in the acute phase of SAH. Because antiplatelet and anticoagulant therapy is not required for balloon-assisted coiling, we were able to embolize the infundibulum in the early stages of SAH. Stent assisted coil embolization necessitates the use of these agents. In their paper, Yu et al. relate a decision to delay treatment of the ruptured infundibulum and were initially unable to identify an aneurysmal rupture site as the source of SAH. Furthermore, based on the patient’s critical condition, the author’s believed the best course of management was to wait for resorption of subarachnoid blood prior to proceeding with intervention [1]. Regardless, the requirement of antiplatelet and anti-coagulant therapy for stent assisted coil embolization complicates management in the acute hemorrhagic setting. Following SAH, the risk of re-rupture peaks on day one at 4%, with 1-2% of patients experiencing rebleeding each day over the course of four weeks following the initial event. With a case fatality rate near 70%, rebleeding following the initial hemorrhagic event results in a dismal prognosis for patients [17]. As such, immediate intervention to secure ruptured infundibula or infundibular aneurysms is necessary to avoid the complications of rebleeding. Stents, flow diverters, and stent assisted coiling requires the added need for antiplatelets, which can complicate management. The case described by Fischer et al. was for stent-assisted coil occlusion of an unruptured aneurysm, and therefore, inapplicable for patients suffering from active subarachnoid hemorrhage [5] The need for antiplatelet therapy for the use of the pipeline flow-diverting system reported by Kameda et al. poses the same problem as stent-assisted coil embolization [18]. As such, stent-assisted coil embolization or the pipeline flow-diversion may be a reasonable choice for unruptured infundibula, however the need for anti-platelet therapy complicates their use in the acute period of SAH and as such, our balloon assisted technique is preferable in cases of recent SAH.
These cases illustrate the importance of establishing appropriate guidelines for the management, and treatment, of intracranial infundibula. In certain populations, these vessels may represent a pre-aneurysmal state rather than a benign anatomic variant. As such, authors have proposed specific groups who may benefit from long-term observation of confirmed infundibula. These groups include patients with a history of intracranial aneurysms, DSA negative SAH, familial occurrence of intracranial aneurysms, or a known disease with an increased propensity for aneurysm formation [19]. In retrospect, our patient’s documented history of familial intracranial aneurysm suggests an increased risk for infundibulum rupture. Therefore, it stands to reason that our patient is at increased risk for the future development of infundibula with subsequent rupture and based on the reported superiority of three-dimensional rotational angiography with volume rendering over two-dimensional digital subtraction angiography in identifying infundibular dilation, our patient may benefit from long term follow up using 3D angiography. Our patient was treated using balloon-assisted coil embolization with good angiographic and clinical outcome. At ten month follow up, digital subtraction angiography revealed a dilatation suspicious for a new aneurysm or possible persistent residual aneurysmal cavity [20]. As such, we advocate the use of this technique as an initial treatment option for patients in the acute phase after SAH attributed to a ruptured PCoA infundibular aneurysm. Due to the possible recurrence verse persistent residual aneurysmal cavity at ten-month follow-up, further study is necessary to ascertain the long-term resiliency of endovascular coil obliteration for the treatment of infundibular aneurysms.
This is the first reported case describing the successful use of balloon assisted coil embolization for the treatment of ruptured infundibular aneurysm during the acute phase of SAH. Balloon-assisted coil embolization is a therapeutic intervention that should be considered for the management of ruptured intracranial infundibular aneurysms and may be an option for the treatment of unruptured infundibula and infundibular aneurysms. The long-term resiliency of endovascular coil obliteration of infundibular aneurysms will need to be further explored.