We wish to present a very unusual case of an adolescent boy who presented with left renal colic and gross hematuria and suspected renal stone. Computed tomogram (CT) of the kidney ureter bladder (KUB) showed a mass lesion in the lower pole of the left kidney and abdominal ultrasound (USS) confirmed it. A contrast enhanced computed tomogram (CECT) scan of the abdomen and pelvis confirmed central necrotic and peripheral enhancing tumor. Abdominal magnetic resonance imaging (MRI) showed a well-defined rounded lesion in the lower pole of the left kidney measuring 28 X 31 X 37 mm and contained mainly cystic areas, which show high signal on T1 and T2 in keeping with a recent hemorrhage. The patient underwent insertion of central venous access line and percutaneous left renal mass biopsies which confirmed pediatric renal cell carcinoma (pRCC). CT chest was normal. The patient underwent left radical nephrectomy and lymph node (LN) sampling uneventfully. Histological examination of the excised specimen confirmed pRCC and LN sampling confirmed one hilar node to be positive. Patient received post-operative chemotherapy, is asymptomatic and well at 2 years follow up.
Keywords: Adolescent, Chemotherapy, Lymph Node, Nephrectomy, Pediatric, Renal Cell Carcinoma, Translocation
Wilms’ tumor or nephroblastoma is the most common renal malignancy in pediatric and adolescent patients, which has well defined therapies and excellent outcomes [1,2,3,4,5]. RCC is the 2nd most common renal malignancy in pediatric and adolescent patients (pRCC), accounting for 2-6% of renal cancers [6]. In contrast to Wilms’ tumor and typical adult RCC, understandings of pRCC are limited, treatment recommendations are based on small retrospective case series and reports, or taken from guidelines for ‘adult’ RCC, pRCC is different from the ‘typical’ adult RCC in both tumor biology and clinical behavior, pRCC is most commonly translocation-type, often harboring chromosomal translocations involving the TFE3 gene at Xp11.2 rather than clear cell RCC typically seen in adults and pRCC have a higher incidence of regional LN involvement yet potential more favorable prognosis when involved nodal disease is completely resected in the absence of distant metastases. Pre-operative transcatheter arterial chemoembolization may be used for very large and/or metastatic RCC [6]. We wish to present our recent experience with a pRCC in an adolescent boy presenting with renal colic and gross hematuria with suspected renal stone disease.
A 13-year-old boy presented with colicky left loin to groin pain and gross hematuria with generalised weakness and weight loss and renal stone disease was suspected. The patient had a history of idiopathic pulmonary inflammatory disease in infancy and recovered uneventfully.
Physical examination showed vital signs within normal limits, gross hematuria was seen and abdominal examination was normal. Initial impression was left renal stone with colic.
An emergency CT KUB showed a well-defined 3.2x2.8cm mass lesion in the lower pole of the left kidney. Trans-abdominal ultrasound confirmed well defined mass lesion (Fig 1 A and B).
A CECT scan of the abdomen and pelvis showed a reasonably well-defined lesion in the lower pole of the left kidney which measured 2.8x2.6x3.7cm (Fig. 2 A and B). This tumor was intensely enhanced after injection of contrast product with hypodense areas of tumor necrosis, hyperdense areas of bleeding and calcifications. It had mixed attenuation with an enhancing rim and a hypoattenuating central region in keeping with fluid. The left kidney was enlarged and moderately hydronephrotic with a thin cortex. The proximal ureter was noted to be prominent and rest of the abdominal organs were normal without para-aortic or mesenteric lymphadenopathy.
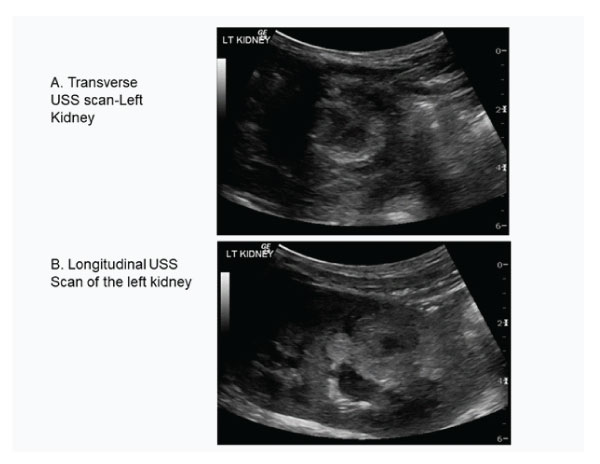
Figure 1A and B : Transverse and longitudinal ultrasound scan showing mass lesion in the lower pole of the left kidney.
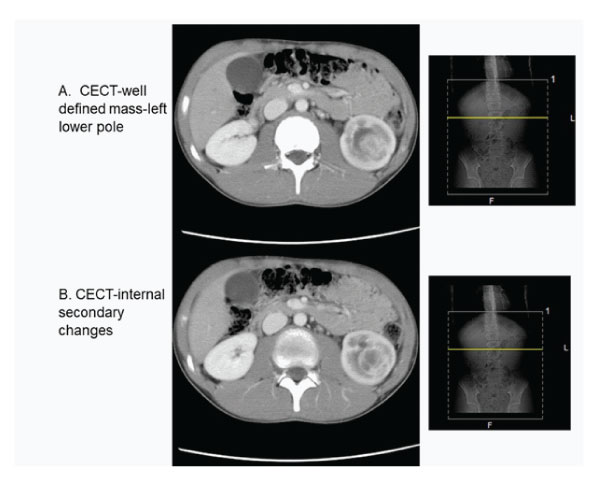
Figure 2A and B : Contrast enhanced CT abdomen showing tumour in the left lower pole of the kidney.
Abdominal MRI using pre and post gadolinium contrast showed a well-defined rounded lesion in the lower pole of the left kidney measured 28x31x37mm and contained mainly cystic areas, which show high signal on T1 and T2 in keeping with a recent hemorrhage (Fig 3 A and B). There were scattered tiny low signal areas in the periphery just under the capsule likely to be hemosiderin deposits. The lesion was causing mass effect on the lower pole calyces which were dilated and blunted. There was no evidence of lymphadenopathy or metastasis in other organs.
The patient underwent examination under anesthesia, insertion of central venous line and percutaneous left renal mass biopsies which confirmed pRCC. Chest radiograph and CT chest were normal.
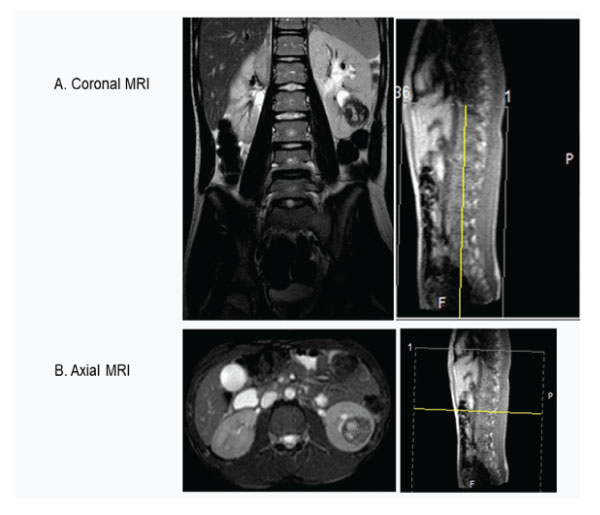
Figure 3A : Coronal and B-Axial MRI scan showing tumour in the left lower pole and no lymph nodes.
The radical left nephrectomy and lymph node sampling were performed uneventfully. The patient was diagnosed with Xp11 translocation on genetic studies and tRCC on the histological examination and none but one hilar lymph node was positive. In view of positive lymph node, single agent chemotherapy typically gemcitabine-based was used initially which was changed to combination chemotherapy gemcitabine/platinum/taxane based therapy by parent’s request as they did not want to take any chance of recurrence as was very precious and only boy in a very large joint family.
During chemotherapy the patient had severe mucositis and developed superior mesenteric artery syndrome due to sudden weight loss and height being higher had height weight mismatch. Initially this was treated with naso-jejunal feeding tube but subsequently percutaneous endoscopic gastrostomy (PEG) tube was inserted and after 6 weeks gastrostomy tube was replaced with jejunal extension (PEG-GJ). Once chemotherapy course was over and he gained weight, PEG-GJ tube was removed. The patient is asymptomatic and doing well at 2 years follow up.
Our case is unusual in that cancer rarely presents with pain and gross hematuria and initial presentation at emergency department suspected renal stone and colic as provisional diagnosis and as per the protocol did CT KUB and before they can refer the patient to pediatric oncology confirmed with USS. Breaking bad news using Spikes protocol was a real challenge from a benign simple stone disease to that of cancer. Moreover, at tumour biopsy our policy is to insert central venous access as the commonest tumour in children is Wilms’ tumour and need for pre-operative chemotherapy was explained but when the pRCC was diagnosed, we have to change the plan and go for a major surgery straight away on an emergency basis. When all preoperative investigations suggested localised disease, hilar lymph node came positive and have to go for adjuvant chemotherapy and as the patient was the only male child in a big joint family, our team was very considerate and empathetic and their request to go for advanced regime from the conventional one was accepted gracefully. pRCC is a unique and distinct adolescent tumour different from other renal tumours. Finally, pRCC is a distinct unique renal tumor as compared to Wilms’ tumor or adult RCC.
Pediatric and adolescent RCC (pRCC) represents a unique therapeutic challenge because of its rarity and its differences from adult RCC or other pediatric renal tumors. The Children's Oncology Group (COG) study AREN03B2 was designed prospectively to collect biological tissue, histologic data, radiographic imaging and surgical data to be used for assignment of patients to a series of therapeutic protocols, to explore novel biological insights, and to help refine future guidelines as there has been no centrally collected and reviewed data. It has systematic, prospective and first largest series of well characterized pRCC tumors, provide insight into the distinctive epidemiology, demographics, histologic heterogeneity, biology, radiographic imaging, and surgical management. They have now reported their experience with pRCC, highlighting the impact on the management of young adult RCC, especially as it relates to the impact of the surgical approach on obtaining lymph nodes [7]
Epidemiological and demographic data, derived from six years of systematic prospective enrolment of 3250 patients with renal tumors from approximately 210 centers, confirms that 3.7% of pediatric renal cancers in those younger than 22 years of age are RCC. There appears to be no gender predilection in pRCC overall, though there is a slight female predominance and right sided lesions for translocation or tRCC specifically. The most common form of pRCC is tRCC, accounting for 47%. tRCC is found in all age groups and all races, first formally recognized by the World health Organization in 2004 as a distinct, typically translocationassociated, RCC with characteristic morphology along with immunohistochemical expression of TFE3 or TFEb [8,9]. Contrary to what may be suspected or reported, RCC genetic cancer predisposition syndromes are not prevalent in this cohort except for an expected association [10].
The use of imaging alone to aid in staging of RCC is well established in adult RCC, but its sensitivity in finding lymph node positivity was poor, missing more than 42% of subsequently histologically confirmed tumor LN metastasis in pRCC, This was the case in our patient as none of the imaging even suspected any LN disease.
Lymphatic Mapping and Surgical Technique:
The day before, or the morning of surgery, all patients underwent preoperative lymphatic mapping with 0.5-1 mCi of Tc99 m injected intra-dermally at the site of primary melanoma. The SLN was identified by the nuclear physician using a handheld gamma probe and then marked on the skin with a permanent marker. Immediately preoperatively 2 ml of Patent Blue dye was injected intra-dermally at the site of primary melanoma scar. All basins identified as radioactive were explored through limited incisions directed by the handheld gamma probe. The SLN was defined as the node with the highest radioactive count. This node was identified and removed, and it was sent in formaldehyde solution directly to the Department of Pathology. Any node(s) with >10% count rate of the most radioactive node was also removed and analysed. Intraoperative SLN histological analysis was not performed on any patient. Patients also underwent a simultaneous wide local excision of the primary melanoma site with margins excised according to current guidelines.
The importance of positive LNs, a common finding with tRCC with rates of 41% in the younger cohort, and up to 5080% in older cohorts, with reports suggesting both a favorable and unfavourable outcome with age as predominate impact factor suggesting more advanced and aggressive tRCC in older and adolescent children. Overall, in pRCC series, approximately 62.5% of tRCC cases present with TNM stage 3 or 4 disease [8]. Poor sensitivity of imaging even in small sized primary tumours combined with the lack of LN sampling over 40 % of cases clearly leads to undertreatment and poor long-term results. This is in sharp contrast with adult RCC where smaller tumours do not have LN positivity. Failure to sample LNs was more likely in lower T-stage cases and those undergoing nephron-sparing surgery. Based in these reasons, a more compulsive approach to LN sampling and dissection seems warranted. It should be re-enforced, as it has been in children with Wilms tumor, that surgical LN sampling is a fundamental part of the surgery for renal tumors in pediatric and adolescent patients.
Radical nephrectomy (RN) is the most common initial surgical treatment in 4/5th cases in pRCC compared with nephron-sparing surgery (NSS) via partial or polar nephrectomy, widely used in adult RCC in remaining 1/5th cases only. However, as our data shows, RN is clearly the predominant surgical approach and NSS should be strictly limited to select few with very small primary tumour and complete local resection with negative resection margins and no lymph nodes positive as the role of NSS is yet to be established in this disease, and given that current adjuvant medical therapies are not typically curative for unresected disease it is important that localized disease be completely resected.
The medical management of tRCC is evolving, predicated on the rationale that tRCC shares similar biology to other RCC variants, conventional chemotherapies, typically gemcitabine-based, have been used with anecdotal response and disease stabilization [8]. Similarly, the medical management of RMC, the most aggressive variant of RCC, may include gemcitabine/platinum/taxane based therapy, as well as biological-based therapy. Evolving evidence of the biological bases of tRCC and RMC will lead to improved targeted therapies.
Published pediatric data suggest that clinical, epidemiological, molecular, histological characteristics, treatment and outcome of pediatric RCC differ from adult RCC and Wilms’ tumour and is unique distinct entity [11,12]. The prognosis for this type of tumor in children is similar to that in adults. According to some authors, the factors influencing the patient's prognosis are the size of the tumor, the age of the patient, complete surgical excision, vascular invasion, and clinical and pathological staging [5].
In conclusion, tRCC is the most common form of pRCC. Although very rare in children, RCC should be part of the diagnostic spectrum for kidney tumors occurring in older children. The type of this tumor still poses problems of differential diagnosis with Wilms’ tumors preoperatively. Nodal disease is common even with small primary tumors. Imaging has a high specificity but relatively low sensitivity for detection of such lymph node metastasis Failure to sample lymph nodes results in incomplete staging and potentially inadequate disease control for younger RCC patients.
Compliance with Ethical Standards
Conflict of Interest
The authors have no conflict of interest to declare. No funding source was involved in this study.
Ethical Approval
All procedures performed on human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from the parents and all the relatives involved prior to all the procedures. Parents and all involved parties were informed about the procedure.